所有图片(1)
About This Item
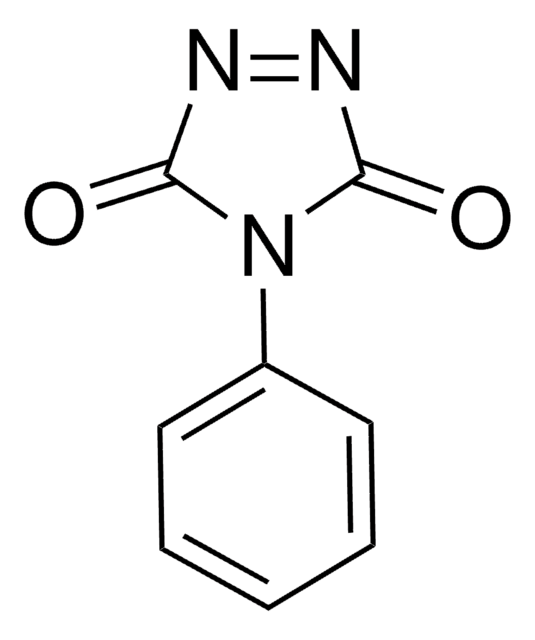
经验公式(希尔记法):
C8H7N3O2
CAS号:
分子量:
177.16
Beilstein:
163361
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
98%
mp
207-209 °C (lit.)
SMILES 字串
O=C1NNC(=O)N1c2ccccc2
InChI
1S/C8H7N3O2/c12-7-9-10-8(13)11(7)6-4-2-1-3-5-6/h1-5H,(H,9,12)(H,10,13)
InChI 密鑰
GOSUFRDROXZXLN-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
4-Phenylurazole undergoes oxidation in the presence of NO2-N2O4 to yield 4-phenyl-1,2,4-trizoline-3,5-dione. It undergoes acetylation reaction with excess acetyl chloride in N,N-dimethylacetamide solution. It polymerizes in the presence of phosgene, terephthaloyl chloride and epichlorohydrin to yield insoluble polymer. It is precursor to the Diels-Alder trapping agent, 4-phenyl-1,2,4-triazoline-3,5-dione. This urazole was recently demonstrated in the traceless, chemoselective labeling of peptides and proteins through electrochemical tyrosine-click (e-Y-CLICK) chemistry.
相關產品
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
A new method for the oxidation of 4-phenylurazole to 4-phenyltriazolinedione.
Mallakpour SE.
Journal of Chemical Education, 69(3), 238-238 (1992)
Bulletin of the Chemical Society of Japan, 61, 3915-3915 (1988)
Synthesis of aliphatic polyamides containing 4-phenylurazole linkages.
Mallakpour SE and Sheikholeslami B.
Polymer International, 48(1), 41-46 (1999)
Journal of the American Chemical Society, 109, 3730-3730 (1987)
Polycondensation Reaction of 4-(4'-N-1, 8-Naphthalimidophenyl)-1, 2, 4-triazolidine-3, 5-dione with Aliphatic Diacid Chlorides.
Mallakpour S and Rafiee Z.
Iranian Polymer Journal, 13, 225-234 (2004)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门