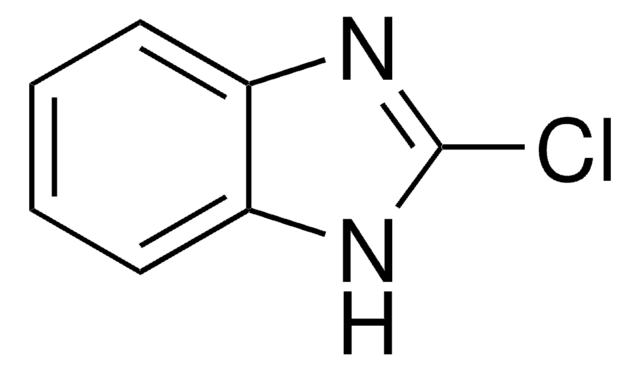
推荐产品
品質等級
化驗
99%
形狀
liquid
折射率
n20/D 1.637 (lit.)
bp
141 °C/30 mmHg (lit.)
mp
21-23 °C (lit.)
密度
1.303 g/mL at 25 °C (lit.)
官能基
chloro
SMILES 字串
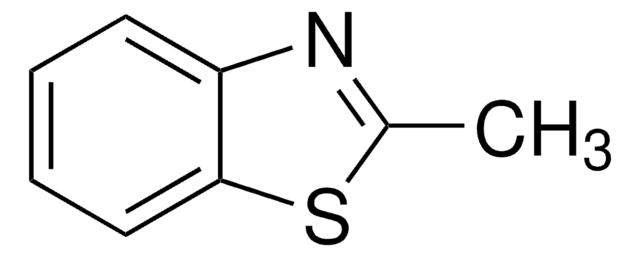
Clc1nc2ccccc2s1
InChI
1S/C7H4ClNS/c8-7-9-5-3-1-2-4-6(5)10-7/h1-4H
InChI 密鑰
BSQLQMLFTHJVKS-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
2-Chlorobenzothiazole was used in the synthesis of:
- (RS)- and (S)-lubeluzole
- (1,3-benzothiazol-2-yl) amino-9-(10H)-acridinone derivatives
- 4H-thieno[2′,3′:4,5]pyrimido[2,1-b]benzothiazole derivatives
訊號詞
Danger
危險分類
Acute Tox. 2 Inhalation - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Irrit. 2
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
230.0 °F - closed cup
閃點(°C)
110 °C - closed cup
個人防護裝備
Eyeshields, Gloves
其他客户在看
Damodara N Kommi et al.
Organic letters, 15(6), 1158-1161 (2013-02-26)
Three new, concise, and protecting group-free synthetic routes for (RS)- and (S)-lubeluzole are reported in higher (46-62%) overall yields compared to the reported procedures (6-35%). The key steps involve C-N bond formation via epoxide aminolysis and nucleophilic substitution of 2-chlorobenzothiazole
Florence Delmas et al.
European journal of medicinal chemistry, 39(8), 685-690 (2004-07-28)
(1,3-Benzothiazol-2-yl) amino-9-(10H)-acridinone derivatives were synthesized via a procedure based on the Ullman reaction and were assessed for their in vitro antileishmanial and anti-HIV activities. Two derivatives, 4-(6-nitro-benzothiazol-2-ylamino)-10H-acridin-9-one and 1-(6-amino-benzothiazol-2-ylamino)-10H-acridin-9-one, revealed a selective antileishmanial activity, mainly due to amastigote-specific toxicity. Results
Synthesis of new thienopyrimidobenzothiazoles and thienopyrimidobenzoxazoles with analgesic and antiinflammatory properties.
Russo F, et al.
European Journal of Medicinal Chemistry, 29(7), 569-578 (1994)
Anita K Kovács et al.
Frontiers in chemistry, 6, 120-120 (2018-05-05)
A general strategy for the synthesis of N-peptide-6-amino-D-luciferin conjugates has been developed. The applicability of the strategy was demonstrated with the preparation of a known substrate, N-Z-Asp-Glu-Val-Asp-6-amino-D-luciferin (N-Z-DEVD-aLuc). N-Z-DEVD-aLuc was obtained via a hybrid liquid/solid phase synthesis method, in which
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门