推荐产品
品質等級
化驗
98%
折射率
n20/D 1.534 (lit.)
bp
225-226 °C (lit.)
密度
1.235 g/mL at 25 °C (lit.)
官能基
acyl chloride
phenoxy
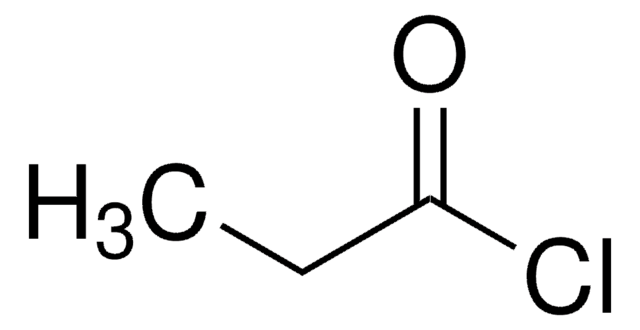
SMILES 字串
ClC(=O)COc1ccccc1
InChI
1S/C8H7ClO2/c9-8(10)6-11-7-4-2-1-3-5-7/h1-5H,6H2
InChI 密鑰
PKUPAJQAJXVUEK-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
Phenoxyacetyl chloride was used in the synthesis of:
- series of macrocyclic bis-β-lactams
- 5-phenyl-6-epiphenoxymethylpenicillin benzyl ester
- N-protected guanosine derivatives, useful in RNA synthesis
- phenyloxyketene, for cycloaddition to imines leading to β-lactams
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B - STOT SE 3
標靶器官
Respiratory system
安全危害
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
Natarajan Arumugam et al.
Medicinal chemistry (Shariqah (United Arab Emirates)), 10(7), 730-737 (2014-02-27)
A series of macrocyclic bis-β-lactams has been synthesized in three good yielding steps using a Staudinger [2+2] cycloaddition reaction of ketene derived from phenoxyacetyl chloride as the key step. The reaction provided a diastereomeric mixture of cis-anti-cis (C2-symmetry) and cis-syn-cis
Tetrahedron, 63, 3380-3380 (2007)
H Vanderhaeghe et al.
Journal of medicinal chemistry, 18(5), 486-490 (1975-05-01)
Cycloaddition of azidoacetyl chloride to benzyl D-5,5-dimethyl-5-phenyl-2-thiazoline-4-carboxylate (1a) gave 5-phenyl-6alpha-azidopenicillanate (2a). By catalytic reduction of 2a and reaction with phenoxyacetyl chloride, 5-phenyl-6-epiphenoxymethylpenicillin benzyl ester (4a) was obtained. Oxidation of 4a gave the sulfoxide 6, which was isomerized in the presence
Yupeng Fan et al.
Organic letters, 6(15), 2555-2557 (2004-07-17)
[reaction: see text] The formation of a guanosine derivative silylated at both the O6 and amino groups was identified by (15)N NMR. This intermediate allows facile reaction with acetyl chloride or phenoxyacetyl chloride to give in high yield the corresponding
Tetrahedron Letters, 48, 1657-1657 (2007)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门