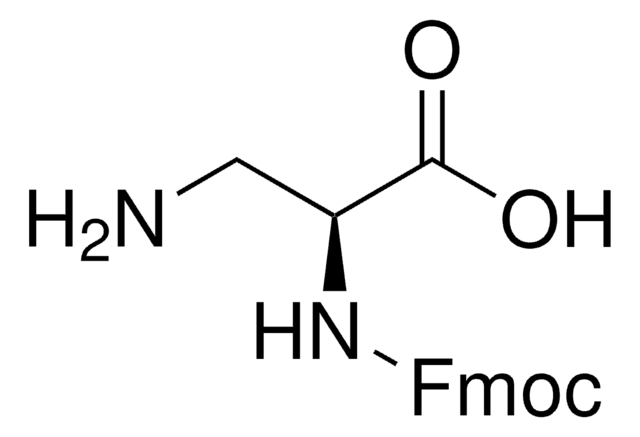
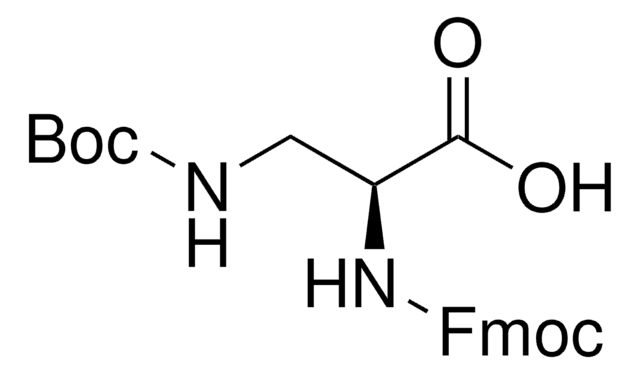
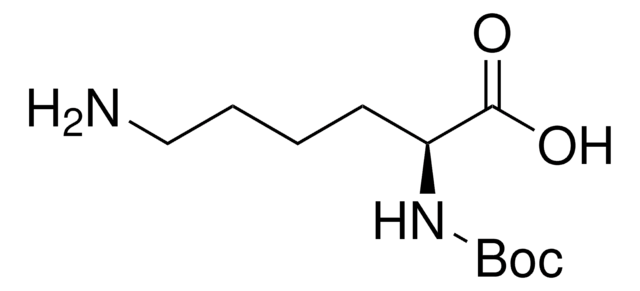
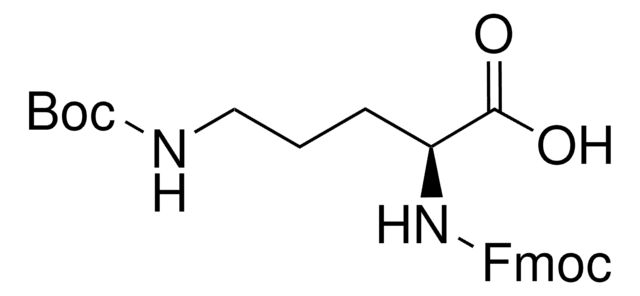
推荐产品
化驗
≥98.0% (TLC)
形狀
solid
光學活性
[α]20/D +5.5±1°, c = 1% in methanol: water (1:1)
反應適用性
reaction type: Boc solid-phase peptide synthesis
雜質
~3% water
mp
210 °C (dec.)
應用
peptide synthesis
SMILES 字串
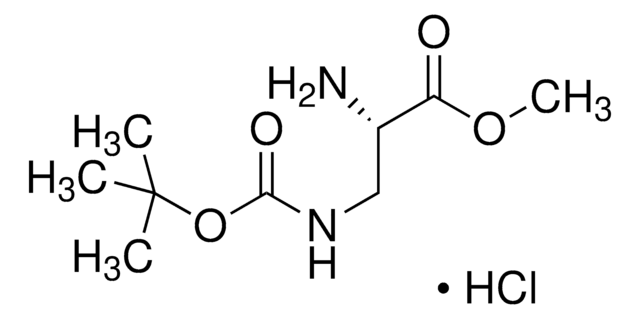
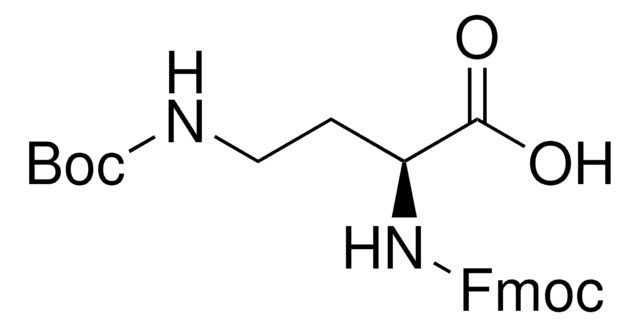
CC(C)(C)OC(=O)N[C@@H](CN)C(O)=O
InChI
1S/C8H16N2O4/c1-8(2,3)14-7(13)10-5(4-9)6(11)12/h5H,4,9H2,1-3H3,(H,10,13)(H,11,12)/t5-/m0/s1
InChI 密鑰
KRJLRVZLNABMAT-YFKPBYRVSA-N
正在寻找类似产品? 访问 产品对比指南
應用
反应物用于:
- 人工合成分子识别基序引导的蛋白质组装
- 具有抗菌和溶血活性的短杆菌肽环状类似物的固相合成
- HCV 蛋白酶抑制剂修饰类似物的合成
- 多肽 V1a 受体激动剂的固相合成
- 脂水界面的定向肽组装
其他說明
DAP 的单保护衍生物;用于例如氨基葡萄糖合成酶抑制剂的合成中, 一种肌球蛋白激酶抑制剂。含金属络合基团多肽的制备。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
N Kucharczyk et al.
Biochemistry, 29(15), 3668-3676 (1990-04-17)
A mechanistic investigation of the inactivation of Escherichia coli glucosamine-6-phosphate synthase by N3-(4-methoxyfumaroyl)-L-2,3-diaminopropionate (FMDP) was undertaken. On the basis of the known participation of the N-terminal cysteine residue in this process [Chmara et al. (1986) Biochim. Biophys. Acta 870, 357;
R Andruszkiewicz et al.
Journal of medicinal chemistry, 33(10), 2755-2759 (1990-10-01)
Six peptide conjugates consisting of either norvaline, methionine, or lysine and N3-(iodoacetyl)-L-2,3-diaminopropanoic acid--a strong, irreversible inactivator of bacterial and fungal glucosamine-6-phosphate synthase--were synthesized and their antibacterial and antifungal activities were evaluated. Antimicrobial potencies of these peptides were correlated with their
J T Hunt et al.
The Biochemical journal, 257(1), 73-78 (1989-01-01)
Although the amino acid residues that are important for peptide substrates of myosin light-chain kinase have been reported, those that are important for peptide inhibitors of this enzyme have not previously been investigated. Synthetic peptides based on the sequence Lys11-Lys12-Arg13-Ala-Ala-Arg16-Ala-Thr-Ser19
F. Ruan et al.
The Journal of Organic Chemistry, 56, 4347-4347 (1991)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门