所有图片(1)
About This Item
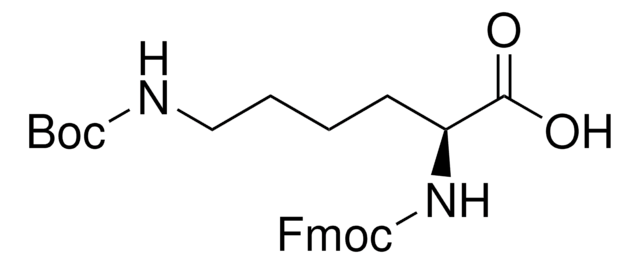
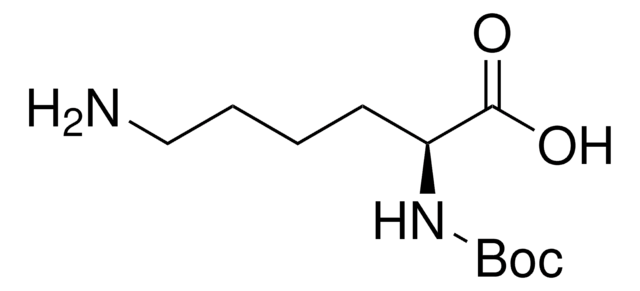
线性分子式:
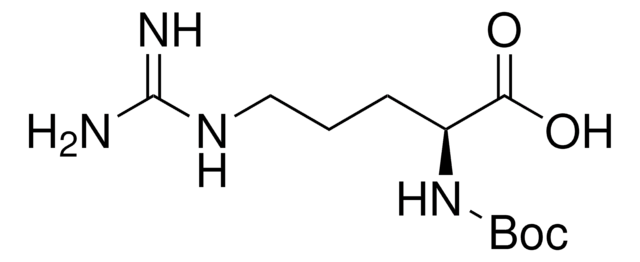
NH2(CH2)4CH(COOH)NHCOOC(CH3)3
CAS号:
分子量:
246.30
Beilstein:
4252546
MDL號碼:
分類程式碼代碼:
12352209
eCl@ss:
32160406
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
99%
形狀
solid
光學活性
[α]/D 4.1 to 5.1°, c = 2 in water
反應適用性
reaction type: Boc solid-phase peptide synthesis
mp
~205 °C (dec.) (lit.)
應用
peptide synthesis
SMILES 字串
CC(C)(C)OC(=O)N[C@@H](CCCCN)C(O)=O
InChI
1S/C11H22N2O4/c1-11(2,3)17-10(16)13-8(9(14)15)6-4-5-7-12/h8H,4-7,12H2,1-3H3,(H,13,16)(H,14,15)/t8-/m0/s1
InChI 密鑰
DQUHYEDEGRNAFO-QMMMGPOBSA-N
正在寻找类似产品? 访问 产品对比指南
應用
Boc-Lys-OH(Nα-叔丁氧羰基-L-赖氨酸)可作为构建单元用于合成:
- 作为生物标记试剂,应用于基于异三功能肽的连接体分子。
- 合成氮杂环和蒽醌的赖氨酸衍生物。
- Boc-Lys(Bn4-DTPA)-OH,作为前体合成含肽的二乙基三胺五乙酸(DTPA)。
- 二茂铁-氨基酸共轭物,用于开发化学战剂(CWA)传感器。
取代透過
产品编号
说明
价格
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Synthesis of Nα-Boc-Nε-tetrabenzyl-DTPA-l-lysine and Nα-Fmoc-Nε-tetra-t-butyl-DTPA-l-lysine, building blocks for solid phase synthesis of DTPA-containing peptides
Davies JS and Al-Jamri L
Journal of Peptide Science, 8(12), 663-670 (2002)
A novel heterotrifunctional peptide-based cross-linking reagent for facile access to bioconjugates. Applications to peptide fluorescent labelling and immobilisation
Clave G, et al.
Organic & Biomolecular Chemistry, 6(17), 3065-3078 (2008)
Synthesis of lysine derivatives containing aza-crown ethers and a chromophore unit
Ossowski T, et al.
Tetrahedron Letters, 46(10), 1735-1738 (2005)
Carla Kühn et al.
Journal of agricultural and food chemistry, 66(26), 6727-6733 (2018-06-09)
Glucosinolates and their breakdown products, especially isothiocyanates (ITCs), are hypothesized to exert a broad range of bioactivities. However, physiological mechanisms are not yet completely understood. In this study, formation of protein conjugates after incubation with benzyl isothiocyanate (BITC) was investigated
Carla Kühn et al.
Molecular nutrition & food research, 62(20), e1800588-e1800588 (2018-08-10)
Different metabolic and excretion pathways of the benzyl glucosinolate breakdown products benzyl isothiocyanate and benzyl cyanide are investigated to obtain information about their multiple fate after ingestion. Detailed focus is on the so far underestimated transformation/excretion pathways-protein conjugation and exhalation.
Global Trade Item Number
| 货号 | GTIN |
|---|---|
| 359688-5G | |
| 359688-1G | 4061831813340 |
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持