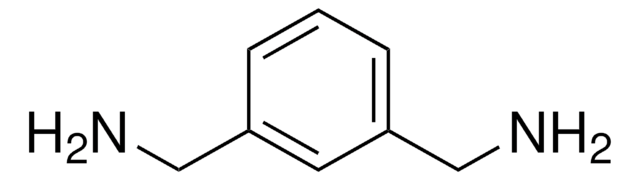
所有图片(2)
About This Item
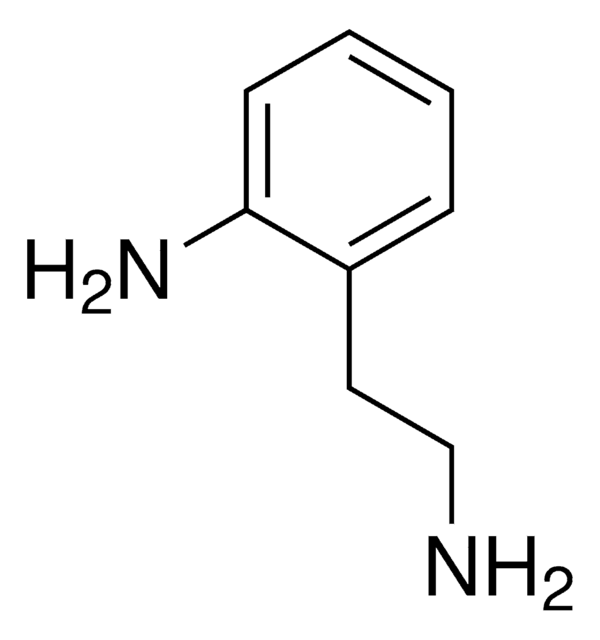
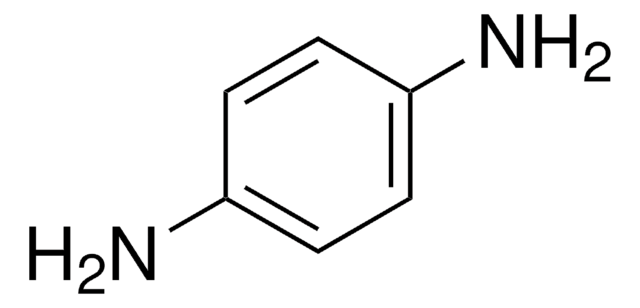
线性分子式:
H2NC6H4CH2CH2NH2
CAS号:
分子量:
136.19
Beilstein:
1099913
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
折射率
n20/D 1.591 (lit.)
bp
103 °C/0.3 mmHg (lit.)
mp
28-31 °C (lit.)
密度
1.034 g/mL at 25 °C (lit.)
官能基
amine
SMILES 字串
NCCc1ccc(N)cc1
InChI
1S/C8H12N2/c9-6-5-7-1-3-8(10)4-2-7/h1-4H,5-6,9-10H2
InChI 密鑰
LNPMZQXEPNWCMG-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
4-(2-氨基乙基)苯胺可通过还原胺化作用与碳水化合物偶联生成修饰的碳水化合物。
應用
4-(2-氨基乙基)苯胺已被用于丝素蛋白的化学改性以调整丝的整体亲水性和结构。它已被用作缩聚反应的试剂。
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
Mihaela Badea et al.
Biosensors & bioelectronics, 18(5-6), 689-698 (2003-04-23)
Glucose oxidase, lactate oxidase, L-aminoacid oxidase and alcohol oxidase were immobilised on new films based on 2,6-dihydroxynaphthalene (2,6-DHN) copolymerised with 2-(4-aminophenyl)-ethylamine (AP-EA) onto the Pt electrodes. The electropolymerisation was performed by cyclic voltammetry. Different scan rates and scan potential ranges
Mitsuo Okada et al.
Plant & cell physiology, 43(5), 505-512 (2002-06-01)
Binding experiments as well as affinity labeling with an (125)I-labeled 2-(4-aminophenyl)ethylamino derivative of N-acetylchitooctaose revealed the presence of high-affinity binding sites/proteins for N-acetylchitooligosaccharide elicitor in the plasma membrane preparation from suspension-cultured carrot cells, barley cells and wheat leaves. Their binding
Chem. Abstr., 116, 106932j-106932j (1992)
H D Grimmecke et al.
Glycoconjugate journal, 15(6), 555-562 (1999-01-09)
Reductive amination of 3-deoxy-D-manno-octulosonic acid (Kdo) with allylamine (AIIN) or 2-(4-aminophenyl)ethylamine (APEA) yields epimer pairs of 2-N-allylamino and 2-N-[2-(4-aminophenyl)ethylamino]-2,3-dideoxy-D-glycero-D-galacto- and -2,3-dideoxy-D-glycero-D-talo-octonic acid. The yields were 50-60% due to reduction of Kdo to the respective polyols as side reaction products. Mass
An improved method for the preparation of derivatives of reducing oligosaccharide with 2-(4-aminophenyl)ethylamine.
L H Semprevivo
Carbohydrate research, 177, 222-227 (1988-06-15)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门