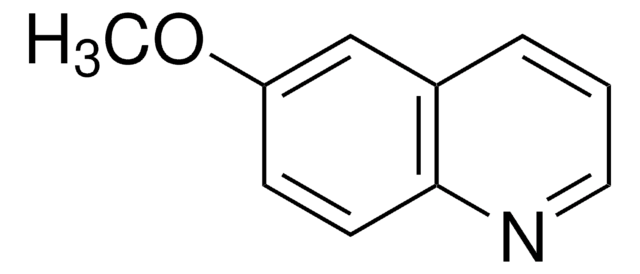
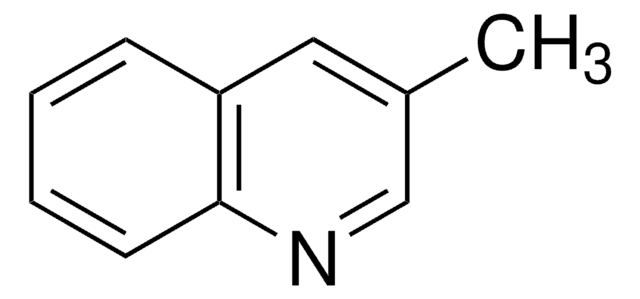
推荐产品
蒸汽密度
>1 (vs air)
化驗
98%
折射率
n20/D 1.614 (lit.)
bp
256-260 °C (lit.)
密度
1.067 g/mL at 20 °C (lit.)
SMILES 字串
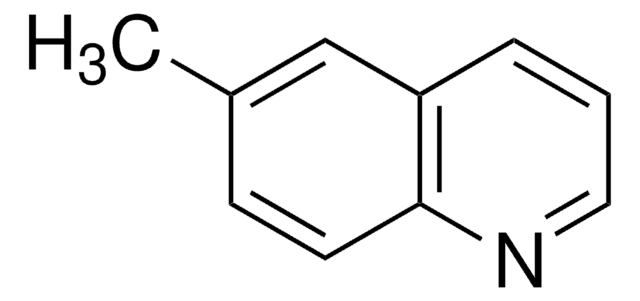
Cc1ccc2ncccc2c1
InChI
1S/C10H9N/c1-8-4-5-10-9(7-8)3-2-6-11-10/h2-7H,1H3
InChI 密鑰
LUYISICIYVKBTA-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
6-Methylquinoline can be used as primary carbon source in culture of Pseudomonas putida QP1. 6-Methylquinoline was used in the synthesis of fluorescent halide-sensitive quinolinium dyes and fluorescent probes for determination of chloride in biological systems.
生化/生理作用
6-Methylquinoline undergoes biodegradation by quinoline-degrading culture of Pseudomonas putida.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Skin Irrit. 2
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Chloride sensitive probes for biological applications.
Geddes CD, et al.
Dyes and Pigments, 48(3), 227-231 (2001)
S Rothenburger et al.
Applied and environmental microbiology, 59(7), 2139-2144 (1993-07-01)
Selective culturing of pseudomonads that could degrade quinoline led to enrichment cultures and pure cultures with expanded substrate utilization and transformation capabilities for substituted quinolines in immobilized and batch cultures. Immobilized cells of the pseudomonad cultures rapidly transformed quinolines to
C D Geddes et al.
Analytical biochemistry, 293(1), 60-66 (2001-05-25)
Three fluorescent halide-sensitive quinolinium dyes have been produced by the reaction of the 6-methylquinoline heterocyclic nitrogen base with methyl bromide, methyl iodide, and 3-bromo-1-propanol. The quaternary salts, unlike the precursor molecule, are readily water soluble and the fluorescence intensity of
Umar Farooq Rizvi et al.
Acta crystallographica. Section C, Crystal structure communications, 64(Pt 10), o547-o549 (2008-10-08)
Molecules of (E)-3-(2-chloro-6-methylquinolin-3-yl)-1-(5-iodo-2-thienyl)prop-2-en-1-one, C(17)H(11)ClINOS, (I), and (E)-3-(2-chloro-6-methylquinolin-3-yl)-1-(5-methyl-2-furyl)prop-2-en-1-one, C(18)H(14)ClNO(2), (II), adopt conformations slightly twisted from coplanarity. Both structures are devoid of classical hydrogen bonds. However, nonclassical C-H...O/N interactions [with C...O = 3.146 (5) A and C...N = 3.487 (3) A] link
C E Scharping et al.
Carcinogenesis, 14(5), 1041-1047 (1993-05-01)
The hepatic microsomal metabolism of the carcinogenic 8-methylquinoline (8MQ) and its noncarcinogenic isomer, 6-methylquinoline (6MQ), were compared for preparations from control rats and rats pretreated with phenobarbital or 3-methylcholanthrene. For each compound the alcohol was the major metabolite, constituting 50-75%
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门