病毒疫苗生产
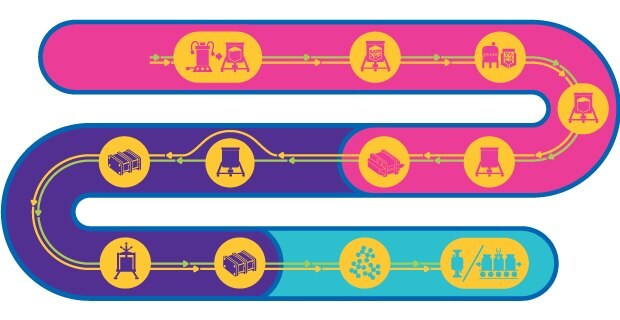
灭活和减毒活病毒的工艺链
相关资源
通过可靠的扩大化优化上游生产力和澄清工艺
为满足生产力要求,必须对针对生产病毒疫苗而开发的上游培养工艺进行优化。该优化涉及澄清步骤,对于去除细胞和细胞碎片并确保稳健的病毒收集至关重要。 然而,只有能够针对预期市场需求进行可靠扩大化的上游工艺才能成功。
通过稳健地去除杂质实现产量和效率目标
来自裂解细胞的核酸是病毒疫苗生产工艺常见的一种污染物。监管规定要求残留的宿主细胞核酸水平需低于10 ng/减毒疫苗剂量。Benzonase®核酸内切酶处理以及随后的切向流过滤是一种稳健而强大的组合,可降解和去除残留的核酸成分。
最大限度提高下游回收率
在浓缩和渗滤过程中,Benzonase®核酸内切酶处理可实现大多数病毒疫苗所需的纯度水平。然而,新一代病毒疫苗(例如日本脑炎病毒(JEV)和登革热病毒(DENV))的纯度目标则需要通过层析来实现。由于每一道生产工艺都必须根据病毒的特征进行定制,因此下游纯化选项的工具箱对于实现所需的纯度、最大限度提高回收率至关重要。
确保患者安全
尽管病毒疫苗是使用减毒病毒生产的,但确保患者的安全仍然是重点。最终的病毒疫苗批量与水相当。因此,在最终配制和灌装完成步骤之前,可使用0.22 µm无菌过滤对疫苗进行灭菌。
工作流程

生物工艺液体细胞培养基和缓冲液
We offer the industry’s highest quality sterile filtered liquid capabilities, supplying ready-to-use cell culture media, buffers, CIP and SIP products from GMP facilities worldwide to optimize your biopharma production.

下游 - 切向流过滤
Achieve yield, efficiency and virus recovery goals while ensuring robust impurity removal

制成品无菌过滤和灌装
Remove cross product contamination concerns while streamlining fill-finishing requirements and complying with current regulatory requirements

如要继续阅读,请登录或创建帐户。
暂无帐户?


