All Photos(2)
About This Item
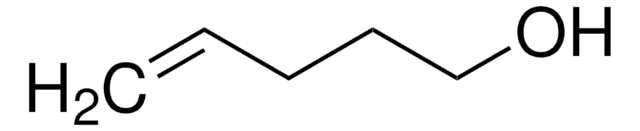
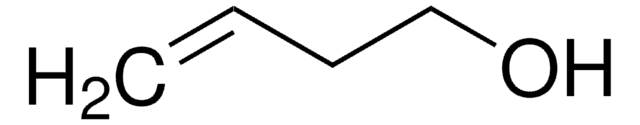
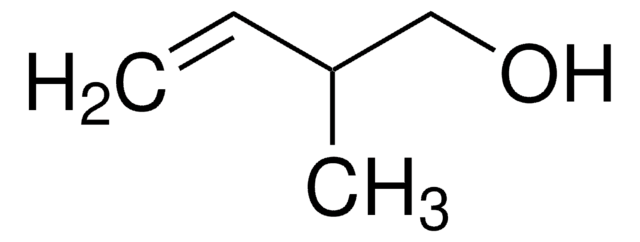
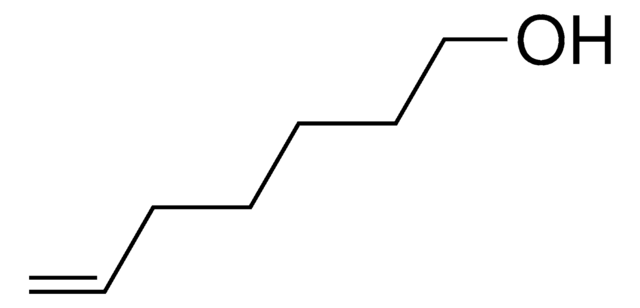
Linear Formula:
CH2=CHCH2CH2OH
CAS Number:
Molecular Weight:
72.11
Beilstein:
1633504
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
refractive index
n20/D 1.421 (lit.)
bp
112-114 °C (lit.)
density
0.838 g/mL at 25 °C (lit.)
functional group
allyl
hydroxyl
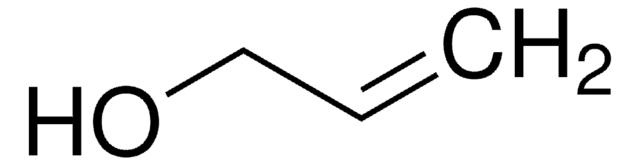
SMILES string
OCCC=C
InChI
1S/C4H8O/c1-2-3-4-5/h2,5H,1,3-4H2
InChI key
ZSPTYLOMNJNZNG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Buten-1-ol is a homoallyl alcohol that can be prepared by the dehydration of 1,4-butanediol using cerium catalyst. The intramolecular hydrogen bonding of 3-buten-1-ol has been studied using FT-IR and 1H NMR spectroscopic data. Its microwave spectrum has been recorded and analyzed. The alkylation reaction of 3-buten-1-ol using titanium-organoaluminum system has been studied. Its gas-phase enthalpy of formation has been reported to be -147.3 ± 1.8kJ mol-1.
Application
Employed in a study of the Mn-catalyzed hydrohydrazination of olefins. Also used in a study of the conversion of propargylic acetates to ethers cataylzed by ferric chloride.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
91.4 °F - closed cup
Flash Point(C)
33 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synlett, 2278-2278 (2006)
The conformational composition of 3-buten-1-ol, the importance of intramolecular hydrogen bonding.
Bakke JM and Bjerkeseth LH.
Journal of Molecular Structure, 470(3), 247-263 (1998)
Transition metal promoted alkylations of unsaturated alcohols: the methylation and ethylation of 3-buten-1-ol using titanium tetrachloride-organoaluminum systems.
Youngblood AV, et al.
Journal of Organometallic Chemistry, 146(3), 221-228 (1978)
Microwave spectrum, intramolecular hydrogen bond, dipole moment and centrifugal distortion of 3-buten-1-ol.
Marstokk KM and Mollendal H.
Acta Chemica Scandinavica, Series A: Physical and Inorganic Chemistry, 35(6), 395-401 (1981)
Jérôme Waser et al.
Journal of the American Chemical Society, 128(35), 11693-11712 (2006-08-31)
The discovery, study, and implementation of the Co- and Mn-catalyzed hydrohydrazination and hydroazidation reactions of olefins are reported. These reactions are equivalent to direct hydroaminations of C-C double bonds with protected hydrazines or hydrazoic acid but are based on a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service