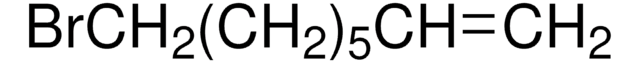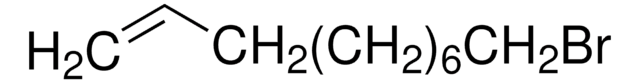253901
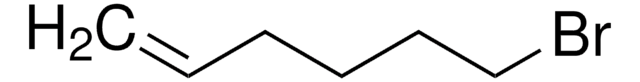
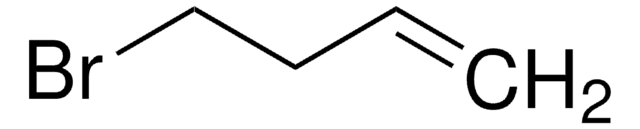
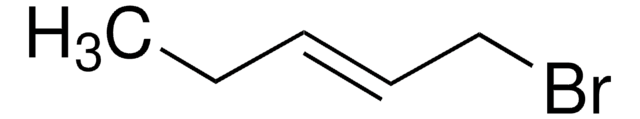
5-Bromo-1-pentene
95%
Synonym(s):
1-Bromo-4-pentene, 4-Pentenyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Br(CH2)3CH=CH2
CAS Number:
Molecular Weight:
149.03
Beilstein:
506077
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.463 (lit.)
bp
126-127 °C/765 mmHg (lit.)
density
1.258 g/mL at 25 °C (lit.)
functional group
alkyl halide
allyl
bromo
storage temp.
2-8°C
SMILES string
BrCCCC=C
InChI
1S/C5H9Br/c1-2-3-4-5-6/h2H,1,3-5H2
InChI key
LPNANKDXVBMDKE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
5-Bromo-1-pentene was used in stereoselective synthesis of 7α-(3-carboxypropyl) estradiol. It was used in preparation of thioacetate 11 of sialic acid having thioglycosidic linkage. It was also used as staring material in recent syntheses of DL-histrionicotoxin and benzophenone-containing fatty acids.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
87.8 °F - closed cup
Flash Point(C)
31 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jun-Ichi Sakamoto et al.
Bioorganic & medicinal chemistry letters, 17(3), 717-721 (2006-11-11)
An efficient synthesis of a series of carbosilane dendrimers uniformly functionalized with alpha-thioglycoside of sialic acid was accomplished. The results of a preliminary study on biological responses against influenza virus sialidases using thiosialoside clusters showed that some of the glycodendrimers
Yonghong Gan et al.
The Journal of organic chemistry, 71(25), 9487-9490 (2006-12-02)
Syntheses of new benzophenone-containing fatty acids (FABPs) 1, 5, and 6 and a new route to FABP 3 are described. Combined with the known 2 and 4, these FABPs comprise a set of photoactivatable fatty acid analogues with the crosslinking
M Adamczyk et al.
Steroids, 62(12), 771-775 (1998-01-22)
Alkylation of 3,17 beta-bis(2-trimethylsilyl)ethoxymethyl-1,3,5(10) estratriene-6-one (2) with 5-bromo-1-pentene using NaHMDS in THF afforded 3,17 beta-bis(2-trimethylsilyl)ethoxymethyl-7-alpha-(4'pentenyl)-1,3,5(10) estratriene-6-one (3) in excellent stereoselectivity (> 95% epimeric excess). Functionalization of the side chain in compound 3 was accomplished via ozonolysis, oxidation and esterification to
Maheswaran S Karatholuvhu et al.
Journal of the American Chemical Society, 128(39), 12656-12657 (2006-09-28)
The synthesis of (+/-)-histrionicotoxin has been achieved in just nine steps using a two-directional synthesis strategy. Key reactions include a two-directional cross-metathesis, a tandem oxime formation/Michael addition/1,4-prototopic shift/[3 + 2]-cycloaddition cascade, a selective Z,Z-bisenyne formation, and a one-pot N-O and
Maher A Qaddoura et al.
International journal of molecular sciences, 10(11), 4772-4788 (2010-01-21)
Several divinylic mesogenic monomers were synthesized based on coupling the monomer 4-(4-pentenyloxy)benzoic acid with chlorohydroquinone, 2,5-dihydroxy- acetophenone, methylhydroquinone or 2-methoxyhydroquinone. This resulted in novel mesogens of phenylene esters with different lateral substituent groups. The effect of the lateral substituent group
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service