512125
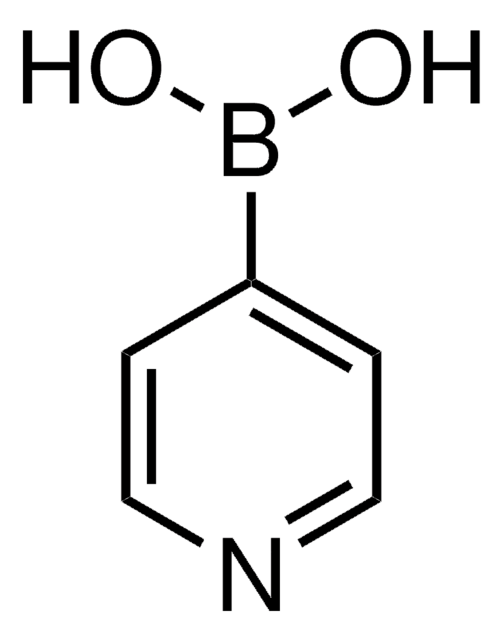
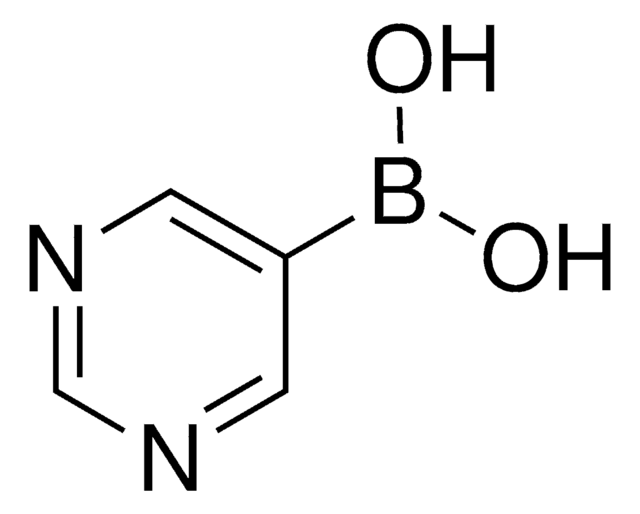
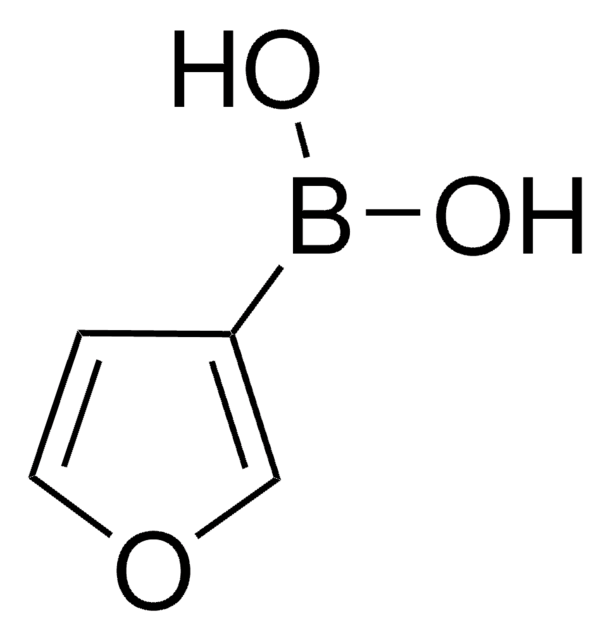
3-Pyridinylboronic acid
≥95.0%
Synonyme(s) :
3-Pyridineboronic acid, 3-Pyridylboronic acid, Dihydroxy(3-pyridyl)borane, Pyridin-3-ylboronic acid
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥95.0%
Forme
solid
Pf
>300 °C (lit.)
Chaîne SMILES
OB(O)c1cccnc1
InChI
1S/C5H6BNO2/c8-6(9)5-2-1-3-7-4-5/h1-4,8-9H
Clé InChI
ABMYEXAYWZJVOV-UHFFFAOYSA-N
Catégories apparentées
Application
- Phosphine-free Suzuki-Miyaura cross-coupling reactions.
- Regioselective Suzuki-Miyaura coupling and tandem palladium-catalyzed intramolecular aminocarbonylation and annulation.
- N-arylation using copper acetylacetonate catalyst.
- Copper-mediated cyanation and regioselective cyanation of electron-rich benzenes.
It can also be used to prepare:
- New linear poly(phenylpyridyl) chains by Suzuki coupling.
- Oligopyridyl foldamers as mimics of a-helix twist.
- Many highly significant therapeutic enzymatic and kinase inhibitors and receptor antagonists.
- Pyridine substituted pyridinium N-(2′-azinyl)aminides by reacting with dibromo pyridinium aminides via Suzuki coupling reaction.
Autres remarques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
This brochure contains a comprehensive selection of boronic acids, boronic acid esters, diboron esters, and transition-metal catalysts useful for the Suzuki–Miyaura coupling reaction
The Suzuki-Miyaura cross-coupling reaction is an important and extensively used reaction in organic chemistry with applications in polymer science and in the fine chemicals and pharmaceutical industries.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique