383449
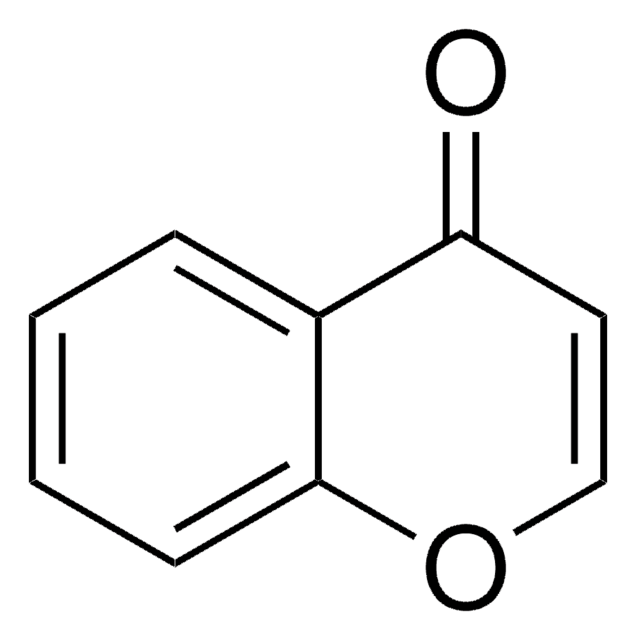
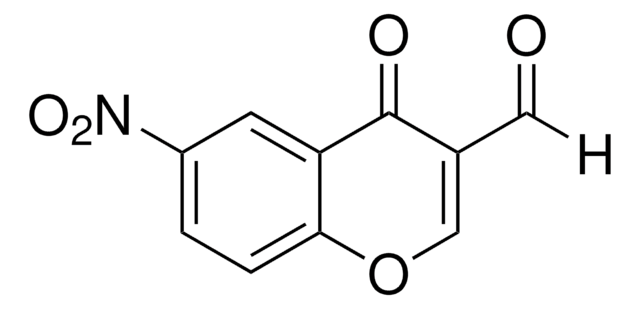
3-Formylchromone
97%
Synonyme(s) :
4-Oxo-4H-1-benzopyran-3-carboxaldehyde
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C10H6O3
Numéro CAS:
Poids moléculaire :
174.15
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Pureté
97%
Forme
solid
Pf
151-153 °C (lit.)
Chaîne SMILES
O=CC1=COc2ccccc2C1=O
InChI
1S/C10H6O3/c11-5-7-6-13-9-4-2-1-3-8(9)10(7)12/h1-6H
Clé InChI
FSMYWBQIMDSGQP-UHFFFAOYSA-N
Informations sur le gène
human ... PTPN1(5770)
Description générale
Electrospray ionization mass spectrometry (ESI-MS) of protonated 3-formylchromone (3-FC) shows loss of H2 as a major fragmentation route to yield a ketene cation, which on reaction with water forms a protonated carboxylic acid. The invivo salubrious effects of 3-FC against nitrosodiethylamine (NDEA) mediated early hepatocellular carcinogenesis has been investigated. Synthesis and characterization of 3-FC and its derivatives has been reported.
Application
3-Formylchromone may be used in the following studies:
- Preparation of library of novel (E)-3-(2-arylcarbonyl-3-(arylamino)allyl)-4H-chromen-4-ones, by three-component domino reactions with (E)-3-(dimethylamino)-1-arylprop-2-en-1-ones and anilines under catalyst-free conditions.
- Facile and ecofriendly synthesis of new chromonyl chalcones.
- Synthesis of 3-(2-hydroxybenzoyl)quinolines and 7H-chromeno[3,2-c]quinolin-7-ones.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Koichi Takao et al.
Bioorganic chemistry, 83, 432-437 (2018-11-15)
A series of eighteen pyrano[4,3-b][1]benzopyranone derivatives (1a-9b) were synthesized, and structure-activity relationships of their monoamine oxidase (MAO) A and B, acetylcholinesterase (AChE), and butyrylcholinesterase (BChE) inhibitory activities were evaluated. Most of the synthesized compounds exhibited weak inhibitory activity toward MAO-A
Pedatsur Neta et al.
Rapid communications in mass spectrometry : RCM, 28(17), 1871-1882 (2014-08-05)
Electrospray ionization mass spectrometry (ESI-MS) of many protonated aldehydes shows loss of CO as a major fragmentation pathway. However, we find that certain aldehydes undergo loss of H2 followed by reaction with water in the collision cell. This complicates interpretation
Andrey S Plaskon et al.
The Journal of organic chemistry, 73(15), 6010-6013 (2008-07-03)
A facile and versatile procedure for the synthesis of 3-(2-hydroxybenzoyl)quinolines and 7H-chromeno[3,2-c]quinolin-7-ones was elaborated on the basis of TMSCl-mediated recyclization of 3-formylchromone with various anilines. Limitations and scope of this methodology were established, and a possible mechanism for the heterocyclizations
Pitchaimani Prasanna et al.
Beilstein journal of organic chemistry, 10, 459-465 (2014-03-13)
The three-component domino reactions of (E)-3-(dimethylamino)-1-arylprop-2-en-1-ones, 3-formylchromone and anilines under catalyst-free conditions afforded a library of novel (E)-3-(2-arylcarbonyl-3-(arylamino)allyl)-4H-chromen-4-ones in good to excellent yields and in a diastereoselective transformation. This transformation generates one C-C and one C-N bond and presumably proceeds
Zeba N Siddiqui et al.
Journal of enzyme inhibition and medicinal chemistry, 27(1), 84-91 (2011-05-27)
A facile and ecofriendly synthesis of new chromonyl chalcones 3a-b from 3-formylchromone 1 and active methyl compounds 2a-b is reported under thermal solvent-free heating condition in good yields. The chromonyl chalcones 3a-b were used as intermediates under green condition for
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique