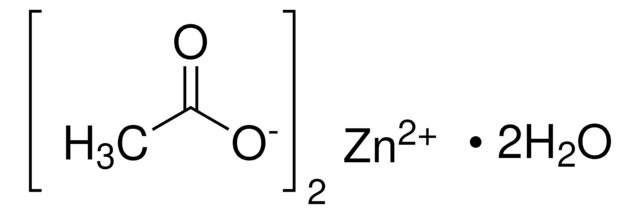
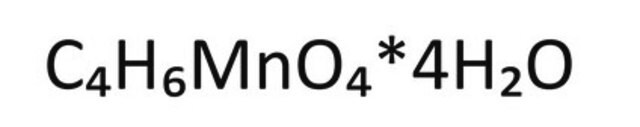229776
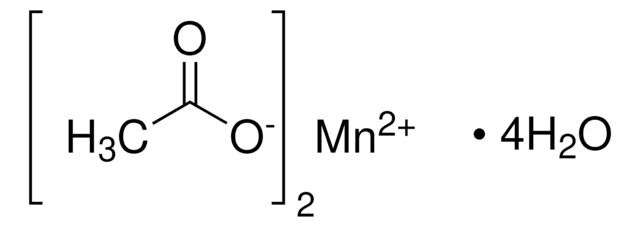
Manganese(II) acetate tetrahydrate
99.99% trace metals basis
Synonyme(s) :
Manganous acetate
About This Item
Produits recommandés
Niveau de qualité
Essai
99.99% trace metals basis
Forme
crystalline powder
Pertinence de la réaction
core: manganese
Pf
>300 °C (lit.)
Densité
1.589 g/mL at 25 °C (lit.)
Chaîne SMILES
[H]O[H].[H]O[H].[H]O[H].[H]O[H].CC(=O)O[Mn]OC(C)=O
InChI
1S/2C2H4O2.Mn.4H2O/c2*1-2(3)4;;;;;/h2*1H3,(H,3,4);;4*1H2/q;;+2;;;;/p-2
Clé InChI
CESXSDZNZGSWSP-UHFFFAOYSA-L
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- A starting material in the synthesis of manganese carboxylates, which are used to prepare manganese oxide thin films by chemical vapor deposition (CVD).
- A precursor in the synthesis of manganese-TiO2 composites by chemical vapor condensation (CVC).These composites are used in the oxidation of NO at low temperatures.
- A precursor in preparation of NCM (LiNi1/3Co1/3Mn1/3O2) powder applicable as cathode material in lithium-ion batteries.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Chronic 3 - STOT RE 2 Inhalation
Organes cibles
Brain
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique