T3580
Toyocamycin
≥98% (HPLC), from Streptomyces rimosus
Sinónimos:
4-Aminopyrrolo[2,3-d]pyrimidine-5-carbonitrile 7-(β-D-ribofuranoside), 7-Deaza-7-cyanoadenosine, NSC 63701, NSC 99843, Neuro 000027, Unamycin B, Vengicide
About This Item
Productos recomendados
origen biológico
Streptomyces rimosus
Nivel de calidad
Ensayo
≥98% (HPLC)
Formulario
solid
solubilidad
DMSO: soluble 0.90-1.10 mg/mL, clear, colorless
H2O: moderately soluble
aqueous acid: moderately soluble
ethanol: moderately soluble
methanol: moderately soluble
espectro de actividad antibiótica
fungi
Modo de acción
DNA synthesis | interferes
temp. de almacenamiento
2-8°C
cadena SMILES
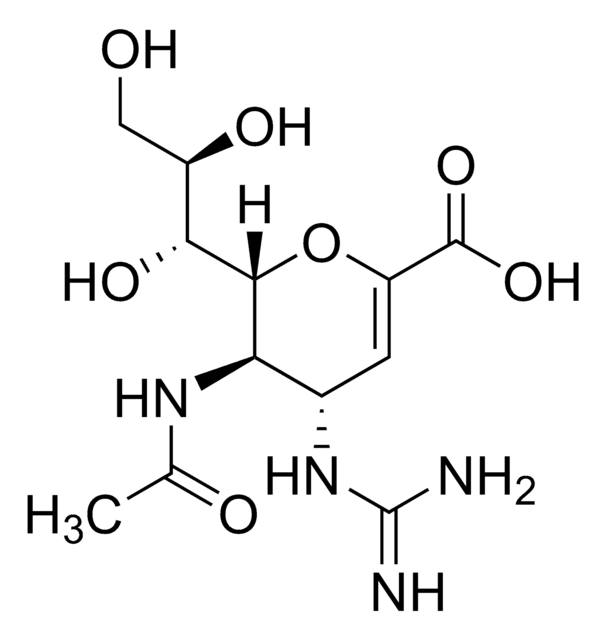
Nc1ncnc2n(cc(C#N)c12)[C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O
InChI
1S/C12H13N5O4/c13-1-5-2-17(11-7(5)10(14)15-4-16-11)12-9(20)8(19)6(3-18)21-12/h2,4,6,8-9,12,18-20H,3H2,(H2,14,15,16)/t6-,8-,9-,12-/m1/s1
Clave InChI
XOKJUSAYZUAMGJ-WOUKDFQISA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Acciones bioquímicas o fisiológicas
Características y beneficios
Nota de preparación
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
We offers many products related to adenosine receptors for your research needs.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico






![PERK Inhibitor I, GSK2606414 GSK2606414 is a cell-permeable, highly potent inhibitor of EIF2AK3/PERK (IC₅₀ = 0.4 nM; [ATP] = 5 µM). Targets PERK in its inactive DFG conformation at the ATP-binding region.](/deepweb/assets/sigmaaldrich/product/structures/180/559/efa716dc-d5fe-4339-a6f0-0103084fc04a/640/efa716dc-d5fe-4339-a6f0-0103084fc04a.png)