SAE0093
Beta-1,4-galactosyltransferase 1
B4GALT1 human recombinant, expressed in HEK 293 cells, 2000 units/mg protein
Sinónimos:
Beta-1,4-GalTase 1, Beta4Gal-T1, UDP-Gal:beta-GlcNAc beta-1,4-galactosyltransferase 1, UDP-galactose:beta-N-acetylglucosamine beta-1,4-galactosyltransferase 1, b4Gal-T1
About This Item
Productos recomendados
recombinante
expressed in HEK 293 cells
Ensayo
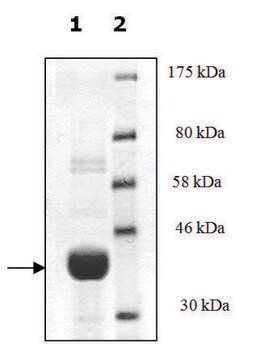
95% (SDS-PAGE)
Formulario
lyophilized powder
actividad específica
2000 units/mg protein
Condiciones de envío
ambient
temp. de almacenamiento
−20°C
Descripción general
Aplicación
Acciones bioquímicas o fisiológicas
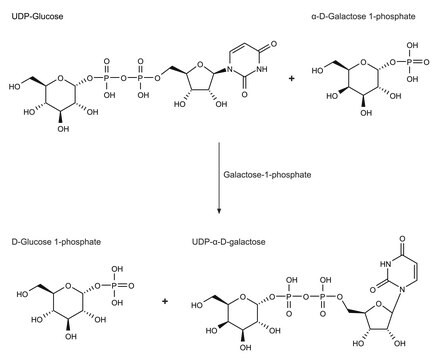
A major function of B4GALT1 is the addition of β(1→4) linked galactose residues to oligosaccharide acceptors with terminal N-acetylglucosamine residues. This is a late elongation step in the N-glycan processing pathway.B4GALT1 enzymatic activity is widely distributed in the vertebrate kingdom, in both mammals and non-mammals, including avians and amphibians.B4GALT1 enzymatic activity has also been demonstrated in a subset of plants which diverged from animals an estimated 1 billion years ago.B4GALT1 interacts with α-lactalbumin (LA), a protein expressed in the mammary gland during lactation, to form the lactose synthase (LS) complex that transfers galactose from UDP-α-D-Gal to glucose, producing the lactose secreted in milk.Defects in B4GALT1 are the cause of congenital disorder of glycosylation type 2D (CDG2D).Glomerular B4GALT1 expression has been found to be increased in IgA nephropathy. IgA binding and IgA-induced mesangial cell phosphorylation of spleen tyrosine kinase and IL-6 synthesis were inhibited by a panel of β(1→4) galactosyltransferase-specific antibodies, which suggests that IgA binds to the catalytic domain of β(1→4) galactosyltransferase.
Definición de unidad
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Glycosyltransferases were initially considered to be specific for a single glycosyl donor and acceptor, which led to the one enzyme-one linkage concept. Subsequent observations have refuted the theory of absolute enzymatic specificity by describing the transfer of analogs of some nucleoside mono- or diphosphate sugar donors.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico