P1431
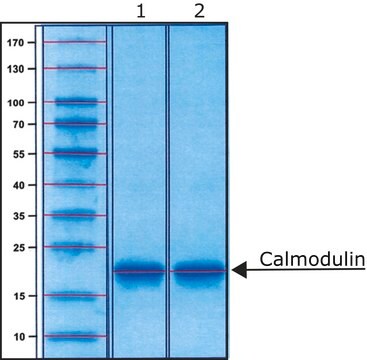
Calmodulin from bovine testes
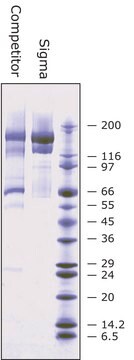
BioUltra, ≥98% (SDS-PAGE), lyophilized powder, essentially salt free
Sinónimos:
CaM, Phosphodiesterase 3′:5′-cyclic nucleotide activator
About This Item
Productos recomendados
origen biológico
bovine testis
Nivel de calidad
Línea del producto
BioUltra
Ensayo
≥98% (SDS-PAGE)
Formulario
lyophilized powder
mol peso
16.79 kDa
condiciones de almacenamiento
(Keep container tightly closed in a dry and well-ventilated place)
técnicas
ligand binding assay: suitable
impurezas
salt, essentially free
Nº de acceso UniProt
aplicaciones
cell analysis
temp. de almacenamiento
−20°C
Información sobre el gen
cow ... CALM3(520277)
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Descripción general
Calmodulin (CaM) is a Ca2+-sensor protein containing four EF-hand motifs that bind to four Ca2+ ions. It is found ubiquitously in all eukaryotes.
Aplicación
- as a component of the reaction mixture in PhosphoSens assay to measure Ca2+/calmodulin-dependent protein kinase II α (CaMKIIα) substrate phosphorylation
- to generate standard curve for the determination of in situ calmodulin concentration in tissues
- as a ligand in radio-ligand binding for studying calmodulin affinity
Acciones bioquímicas o fisiológicas
Producto relacionado
anticuerpo
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico