N9284
Nitroreductase from Escherichia coli
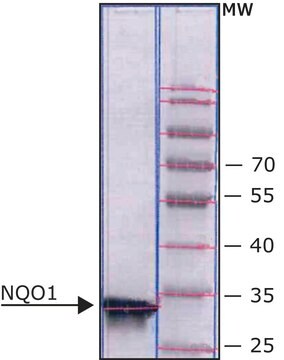
≥90% (SDS-PAGE), recombinant, expressed in E. coli
Sinónimos:
NTRA
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Productos recomendados
recombinante
expressed in E. coli
Nivel de calidad
Ensayo
≥90% (SDS-PAGE)
Formulario
lyophilized powder
actividad específica
≥100 units/mL
mol peso
monomer 24000
características de los productos alternativos más sostenibles
Waste Prevention
Safer Solvents and Auxiliaries
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Nº de acceso UniProt
categoría alternativa más sostenible
Condiciones de envío
wet ice
temp. de almacenamiento
−20°C
Información sobre el gen
Escherichia coli K12 ... nfsB(945483)
Descripción general
Nitroreductase is a flavoprotein and is encoded by the NfsB gene. It comprises a dimer, with 217 amino acids and active site in each subunit. The structure has FMN and the substrate bound to the enzyme.
Aplicación
Nitroreductase from Escherichia coli has been used in the conjugation generation with pig liver esterase (PLE). It has also been used in chemiluminescence response studies with probes HyCL-3 and HyCL-4-AM in rat liver microsomes.
Acciones bioquímicas o fisiológicas
Nitroreductase (NTR) catalyzes the reduction of nitroaromatic substrates and quinones. The mutant F124K of NTR is useful in cancer therapy and improves sensitization of drug CB1954.
Nitroreductase increases the sensitivity of organisms to nitro-containing drugs such as metronidazole by converting the nitro group to a cytotoxic nitro radical.
Nitroreductases can play a crucial role in redox systems via NADPH or NADH as a reductant.
Shows ability to reduce quinines. Enzyme for activating prodrugs in antibody directed enzyme prodrug therapy.
Propiedades físicas
Lyophilized powder containing PBS. Does not contain BSA as excipient
Definición de unidad
One unit will reduce one μmole of Cytochrome C per minute in the presence of Menadione and NADH at pH 7.4 at 37 °C.
Nota de preparación
Produced using animal component-free materials.
Código de clase de almacenamiento
13 - Non Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Dual enzyme-responsive ?turn-on? fluorescence sensing systems based on in situ formation of 7-hydroxy-2-iminocoumarin scaffolds
Debieu, S and Romieu A
Organic & Biomolecular Chemistry, 13(41), 10348-10361 (2015)
Chih-Chen Chen et al.
Food chemistry, 135(4), 2708-2713 (2012-09-18)
Nitroreductases (Nrs) play important roles in redox system via NADPH or NADH as a reductant. A TcNr cDNA encoding a putative Nr was cloned from Taiwanofungus camphorata. A 3-D structural model of the TcNr has been created based on the
Mansooreh Jaberipour et al.
Biochemical pharmacology, 79(2), 102-111 (2009-08-12)
Prodrug activation gene therapy for cancer involves expressing prodrug-activating enzymes in tumour cells, so they can be selectively killed by systemically administered prodrug. For example, Escherichia colinfsB nitroreductase (E.C. 1.6.99.7)(NTR), sensitises cells to the prodrug CB1954 (5-[aziridin-1-yl]-2,4-dinitrobenzamide), which it converts
Bharat Bhushan et al.
Biochemical and biophysical research communications, 322(1), 271-276 (2004-08-18)
Previously, we reported that a salicylate 1-monooxygenase from Pseudomonas sp. ATCC 29352 biotransformed CL-20 (2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaaza-isowurtzitane) (C(6)H(6)N(12)O(12)) and produced a key metabolite with mol. wt. 346 Da corresponding to an empirical formula of C(6)H(6)N(10)O(8) which spontaneously decomposed in aqueous medium to
M J Lemmon et al.
Gene therapy, 4(8), 791-796 (1997-08-01)
A fundamental obstacle in gene therapy for cancer treatment is the specific delivery of an anticancer gene product to a solid tumor. Although several strategies exist to control gene expression once a vector is directly introduced into a tumor, as
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico![Nitrate Reductase (NAD[P]H) from Aspergillus niger lyophilized powder, ≥300 units/g solid](/deepweb/assets/sigmaaldrich/product/images/309/282/2a67ae4d-ca55-4f0b-96ec-34748ff8a21e/640/2a67ae4d-ca55-4f0b-96ec-34748ff8a21e.jpg)