M6626
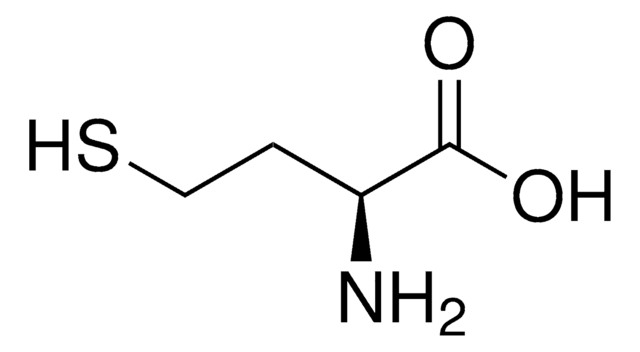
S-Methyl-L-cysteine
suitable for ligand binding assays
Sinónimos:
(R)-2-Amino-3-(methylmercapto)propionic acid, SMLC
About This Item
Productos recomendados
product name
S-Methyl-L-cysteine, substrate for methionine sulfoxide reductase A
Nivel de calidad
formulario
powder
técnicas
ligand binding assay: suitable
aplicaciones
peptide synthesis
temp. de almacenamiento
−20°C
cadena SMILES
CSC[C@H](N)C(O)=O
InChI
1S/C4H9NO2S/c1-8-2-3(5)4(6)7/h3H,2,5H2,1H3,(H,6,7)/t3-/m0/s1
Clave InChI
IDIDJDIHTAOVLG-VKHMYHEASA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
- In-vitro antioxidant and anti-inflammatory potential along with p.o. pharmacokinetic profile of key bioactive phytocompounds of Snow Mountain Garlic: a comparative analysis vis-à-vis normal garlic.: This study examines the antioxidant and anti-inflammatory properties of S-Methyl-L-cysteine found in Snow Mountain Garlic, providing insights into its potential therapeutic applications (Kaur et al., 2024).
- Acidification and tissue disruption affect glucosinolate and S-methyl-l-cysteine sulfoxide hydrolysis and formation of amines, isothiocyanates and other organosulfur compounds in red cabbage (Brassica oleracea var. capitata f. rubra).: The research highlights how acidification and tissue disruption influence the hydrolysis of S-Methyl-L-cysteine sulfoxide, impacting the formation of various bioactive compounds in red cabbage (Hanschen, 2024).
- Selective cycloaddition of ethylene oxide to CO(2) within the confined space of an amino acid-based metal-organic framework.: This research explores the use of an S-Methyl-L-cysteine-based metal-organic framework for the selective cycloaddition of ethylene oxide to CO2, demonstrating its potential in green chemistry and materials science (Bilanin et al., 2023).
Acciones bioquímicas o fisiológicas
When used as a dietary supplement in the Drosphilia model of PD, SMLC increases the efficacy of the MRSA catalytic antioxidant system by providing additional substrate available leading to increased resistance to oxidative stress.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico