M2525
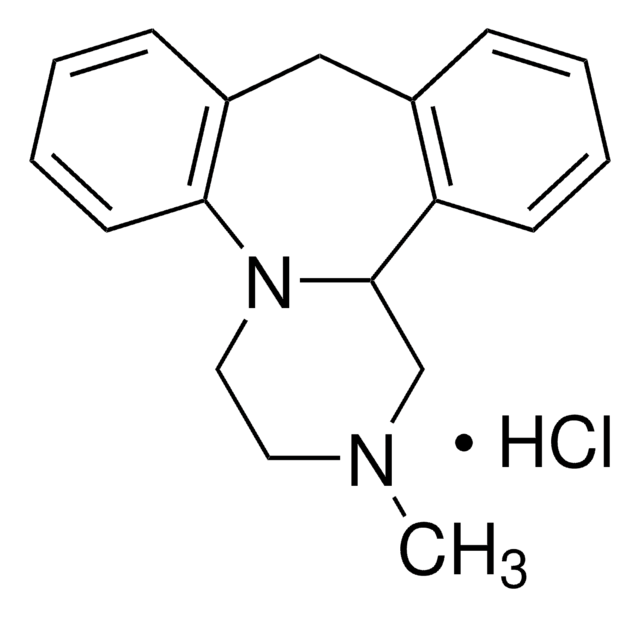
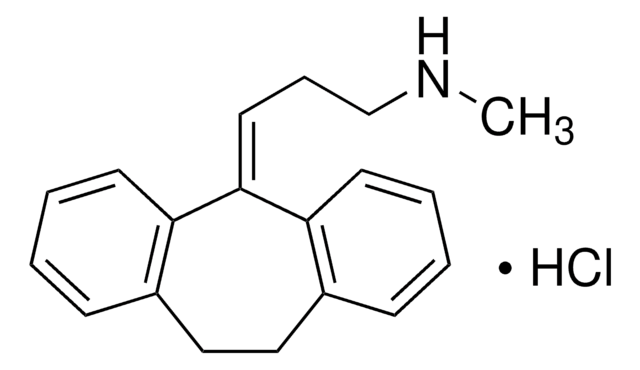
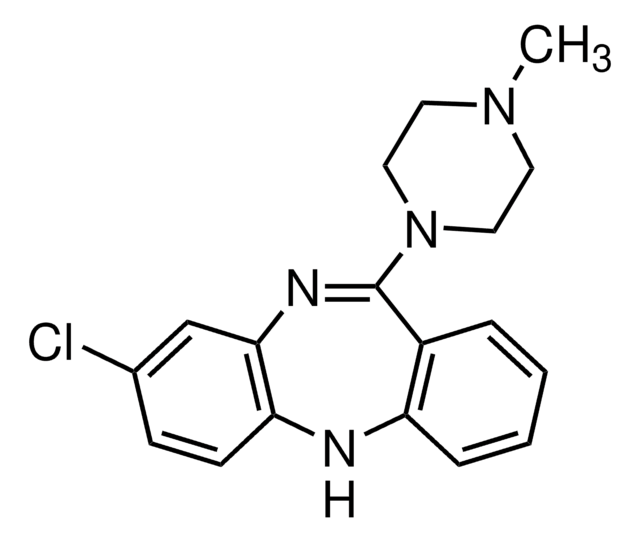
Mianserin hydrochloride
Sinónimos:
1,2,3,4,10,14b-Hexahydro-2-methyl-dibenzo[c,f]pyrazino[1,2-a]azepine hydrochloride
About This Item
Productos recomendados
solubilidad
H2O: 3.4 mg/mL
ethanol: 5.6 mg/mL
emisor
Organon
temp. de almacenamiento
2-8°C
cadena SMILES
Cl.CN1CCN2C(C1)c3ccccc3Cc4ccccc24
InChI
1S/C18H20N2.ClH/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19;/h2-9,18H,10-13H2,1H3;1H
Clave InChI
YNPFMWCWRVTGKJ-UHFFFAOYSA-N
Información sobre el gen
human ... ADRA2A(150) , ADRA2B(151) , ADRA2C(152) , HRH1(3269) , HTR2A(3356) , HTR2B(3357) , HTR2C(3358)
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
- as a reversible antagonist for serotonergic -protein coupled receptor (GPCR) - G-protein protein-coupled receptor (S7.1R)
- as an antidepressant in hippocampal astrocytes to test its effect on brain-derived neurotrophic factor (BDNF) mRNA expression
- as a 5-hydroxytryptamine (5-HT) receptor antagonist to study its effect on serotonin modulation
Acciones bioquímicas o fisiológicas
Características y beneficios
Nota de preparación
Aplicación
Palabra de señalización
Warning
Frases de peligro
Clasificaciones de peligro
Acute Tox. 4 Oral
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Chromatograms
application for HPLCapplication for HPLCapplication for HPLCapplication for HPLCMostrar másNuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico