D9568
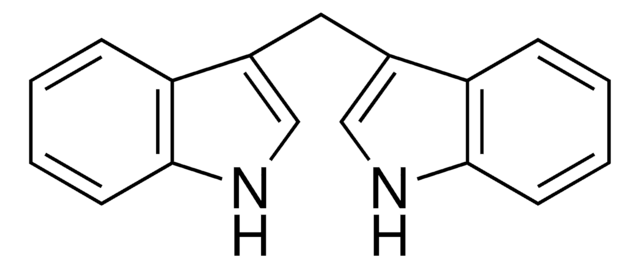
3,3′-Diindolylmethane
≥98% (HPLC)
Sinónimos:
DIM
About This Item
Productos recomendados
Nivel de calidad
Análisis
≥98% (HPLC)
formulario
powder
temp. de almacenamiento
2-8°C
cadena SMILES
C(c1c[nH]c2ccccc12)c3c[nH]c4ccccc34
InChI
1S/C17H14N2/c1-3-7-16-14(5-1)12(10-18-16)9-13-11-19-17-8-4-2-6-15(13)17/h1-8,10-11,18-19H,9H2
Clave InChI
VFTRKSBEFQDZKX-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
- 3,3′-Diindolylmethane inhibits Th17 cell differentiation via impairing IRF-7-mediated plasmacytoid dendritic cell activation in imiquimod-induced psoriasis mice.: The research indicates that 3,3′-Diindolylmethane can effectively inhibit Th17 cell differentiation, offering a potential therapeutic approach for treating psoriasis by targeting plasmacytoid dendritic cell pathways (Rasool et al., 2024).
- Protective effect of diindolylmethane-enriched dietary cabbage against doxorubicin-induced cardiotoxicity in mice.: This study highlights the cardioprotective effects of a diindolylmethane-enriched diet in mice, offering a dietary approach to mitigate the cardiotoxic effects of doxorubicin, a common chemotherapeutic agent (Natesh et al., 2024).
- Nanoformulated 3′-diindolylmethane modulates apoptosis, migration, and angiogenesis in breast cancer cells.: The investigation into nanoformulated 3′-diindolylmethane shows it significantly influences apoptosis, migration, and angiogenesis, suggesting its utility in targeted cancer therapies (Harakeh et al., 2024).
- Design, synthesis, and biological evaluation of 3,3′-diindolylmethane N-linked glycoconjugate as a leishmanial topoisomerase IB inhibitor with reduced cytotoxicity.: Research presents a synthesized glycoconjugate of 3,3′-Diindolylmethane as an effective inhibitor of leishmanial topoisomerase IB, demonstrating reduced cytotoxicity and potential as a therapeutic agent (Kour et al., 2023).
Acciones bioquímicas o fisiológicas
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Aquatic Chronic 4 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico