I7256
Indole-3-carbinol
Sinónimos:
3-Indolemethanol
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
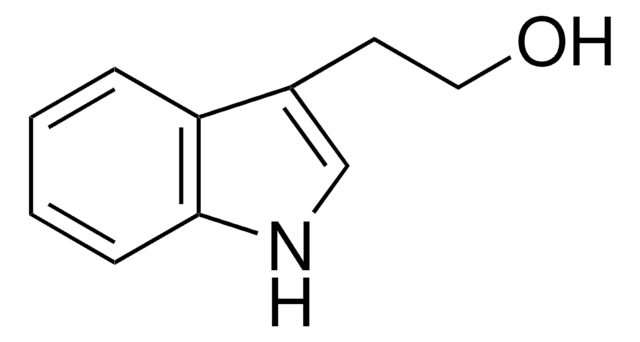
C9H9NO
Número de CAS:
Peso molecular:
147.17
Número CE:
Número MDL:
Código UNSPSC:
12352005
ID de la sustancia en PubChem:
NACRES:
NA.25
Productos recomendados
Nivel de calidad
mp
96-99 °C (lit.)
temp. de almacenamiento
2-8°C
cadena SMILES
OCc1c[nH]c2ccccc12
InChI
1S/C9H9NO/c11-6-7-5-10-9-4-2-1-3-8(7)9/h1-5,10-11H,6H2
Clave InChI
IVYPNXXAYMYVSP-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Indole-3-carbinol is a novel secondary plant metabolite produced in cruciferous vegetables, such as cabbage, cauliflower and brussels sprouts.
Aplicación
Indole-3-carbinol has been used for encapsulation with poly-lactic-co-glycolic acid (PLGA), to study its in-vitro anti-cancerogenic effects on breast adenocarcinoma epithelial (MCF7), colon adenocarcinoma epithelial (Caco2), prostate carcinoma epithelial (PC3) cells. It has also been used as a cytochrome P4501A (CYP1A) inducer.
Acciones bioquímicas o fisiológicas
Indole-3-carbinol (I3C) activates aryl hydrocarbon receptor (AhR) and induces G1 cell cycle arrest and apoptosis. Thus, it acts as a potential anti-cancer agent. In addition, it induces estradiol metabolism by stimulating cytochrome P450 enzymes. Therefore, I3C is considered to be a potent chemotherapeutic for various types of cancer including, breast, prostate, colon cancer, and leukemia.
Inhibits cancinogenesis at the initiation stage. Has be shown to inhibit carcinogenesis in several animal species, but it enhances tumor incidence if administered at a post-initiation stage. Found in cruciferous vegetables.
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin.
Bjeldanes LF, et al.
Proceedings of the National Academy of Sciences of the USA, 88(21), 9543-9547 (1991)
Jing-Ru Weng et al.
Cancer letters, 262(2), 153-163 (2008-03-04)
During the course of oncogenesis and tumor progression, cancer cells constitutively upregulate signaling pathways relevant to cell proliferation and survival as a strategy to overcome genomic instability and acquire resistance phenotype to chemotherapeutic agents. In light of this clinical and
G S Bailey et al.
Journal of the National Cancer Institute, 78(5), 931-934 (1987-05-01)
Indole-3-carbinol (I3C), a natural constituent of cruciferous vegetables, is an inhibitor in several experimental animal models of carcinogenesis by polynuclear aromatic hydrocarbons or aflatoxin B1 (AFB1) when administered prior to or during carcinogen exposure. For assessment of the postinitiation effects
H L Bradlow et al.
Annals of the New York Academy of Sciences, 889, 204-213 (2000-02-11)
Previous studies from this laboratory have suggested that 2-hydroxyestrone is protective against breast cancer, whereas the other principal metabolite, 16 alpha-hydroxyestrone, and the lesser metabolite quantitatively, 4-hydroxyestrone, are potent carcinogens. Attempts to directly decrease the formation of the 16-hydroxylated metabolite
PLGA encapsulation and radioiodination of indole-3-carbinol: investigation of anticancerogenic effects against MCF7, Caco2 and PC3 cells by in vitro assays
Yildiz G, et al.
J. Radioanal. Nucl. Chem., 311(2), 1043-1052 (2017)
Artículos
NF-κB and Inflammation
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico