C4879
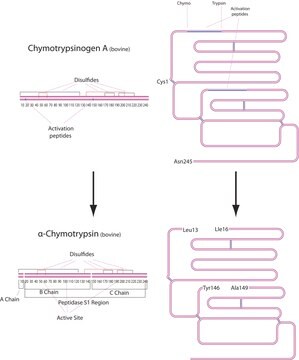
α-Chymotrypsinogen A from bovine pancreas
essentially salt-free, lyophilized powder
Sinónimos:
chymotrypsin A zymogen
About This Item
Productos recomendados
origen biológico
bovine pancreas
Nivel de calidad
tpo
Type II
formulario
essentially salt-free, lyophilized powder
actividad específica
≥40 units/mg solid
mol peso
25,656 Da by calculation
purificado por
6× crystallization
solubilidad
1 mM HCl: soluble 10 mg/mL, clear, colorless
Nº de acceso UniProt
actividad extraña
α-chymotrypsin ≤1 U/mg (prior to activation by trypsin)
temp. de almacenamiento
−20°C
Información sobre el gen
cow ... CTRB1(618826)
Categorías relacionadas
Descripción general
Aplicación
Acciones bioquímicas o fisiológicas
Definición de unidad
Otras notas
Aplicación
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Separation of Ribonuclease A from bovine pancreas, Type I-A, powder, ≥60% RNase A basis (SDS-PAGE), ≥50 Kunitz units/mg protein; α-Chymotrypsinogen A from bovine pancreas, essentially salt-free, lyophilized powder; Cytochrome c from bovine heart, ≥95% based on Mol. Wt. 12,327 basis; Lysozyme from chicken egg white, lyophilized powder, protein ≥90 %, ≥40,000 units/mg protein
Separation of Ribonuclease A from bovine pancreas, Type I-A, powder, ≥60% RNase A basis (SDS-PAGE), ≥50 Kunitz units/mg protein; α-Chymotrypsinogen A from bovine pancreas, essentially salt-free, lyophilized powder; Cytochrome c from bovine heart, ≥95% based on Mol. Wt. 12,327 basis; Lysozyme from chicken egg white, lyophilized powder, protein ≥90 %, ≥40,000 units/mg protein
Separation of Ribonuclease A from bovine pancreas, Type I-A, powder, ≥60% RNase A basis (SDS-PAGE), ≥50 Kunitz units/mg protein; α-Chymotrypsinogen A from bovine pancreas, essentially salt-free, lyophilized powder; Cytochrome c from bovine heart, ≥95% based on Mol. Wt. 12,327 basis; Lysozyme from chicken egg white, lyophilized powder, protein ≥90 %, ≥40,000 units/mg protein
Separation of Ribonuclease A from bovine pancreas, Type I-A, powder, ≥60% RNase A basis (SDS-PAGE), ≥50 Kunitz units/mg protein; α-Chymotrypsinogen A from bovine pancreas, essentially salt-free, lyophilized powder; Cytochrome c from bovine heart, ≥95% based on Mol. Wt. 12,327 basis; Lysozyme from chicken egg white, lyophilized powder, protein ≥90 %, ≥40,000 units/mg protein
Chromatograms
application for HPLCNuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico