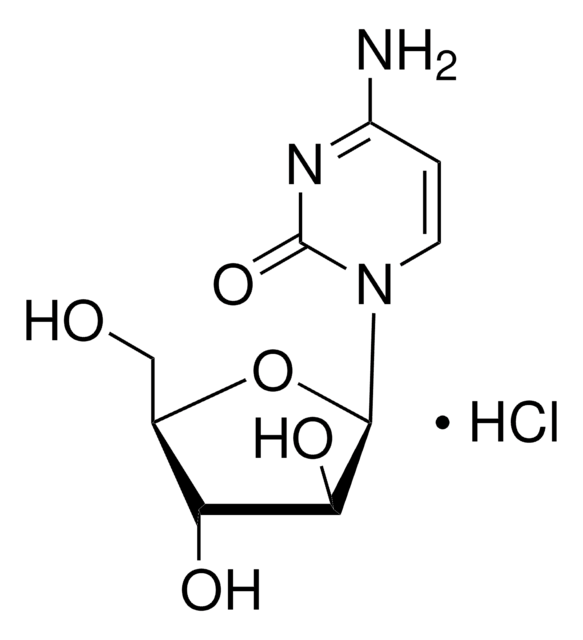
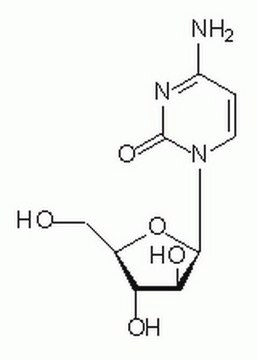
C1768
Cytarabine
≥90% (HPLC), crystalline, DNA replication inhibitor
Sinónimos:
(β-D-Arabinofuranosyl)cytosine, Ara-C, Arabinocytidine, Arabinosylcytosine, Cytarabine, Cytosine arabinoside
About This Item
Productos recomendados
Nombre del producto
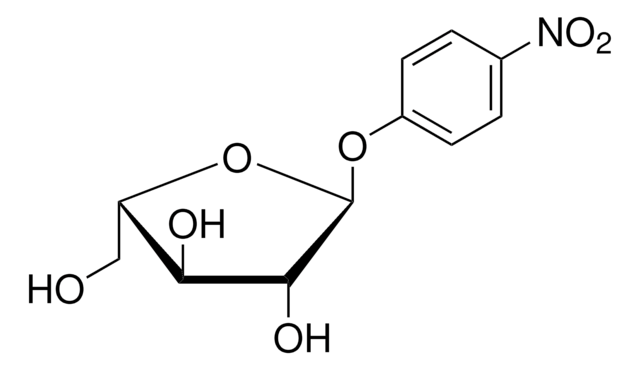
Cytosine β-D-arabinofuranoside, crystalline, ≥90% (HPLC)
Ensayo
≥90% (HPLC)
Formulario
crystalline
temp. de almacenamiento
2-8°C
cadena SMILES
NC1=NC(=O)N(C=C1)[C@@H]2O[C@H](CO)[C@@H](O)[C@@H]2O
InChI
1S/C9H13N3O5/c10-5-1-2-12(9(16)11-5)8-7(15)6(14)4(3-13)17-8/h1-2,4,6-8,13-15H,3H2,(H2,10,11,16)/t4-,6-,7+,8-/m1/s1
Clave InChI
UHDGCWIWMRVCDJ-CCXZUQQUSA-N
Información sobre el gen
human ... POLA1(5422) , POLA2(23649) , POLB(5423) , POLD1(5424) , POLD2(5425) , POLD3(10714) , POLD4(57804) , POLE(5426) , POLE2(5427) , POLE3(54107) , PRIM1(5557) , PRIM2(5558)
mouse ... Cda(72269)
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Acciones bioquímicas o fisiológicas
Características y beneficios
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Repr. 2 - Skin Sens. 1
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Neoplastic cells are highly dependent on the de novo synthesis of nucleotides to maintain sufficient pools to support DNA replication and the production of RNA.
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
Contenido relacionado
Apoptosis, or programmed cell death (PCD), is a selective process for the removal of unnecessary, infected or transformed cells in various biological systems. As it plays a role in the homeostasis of multicellular organisms, apoptosis is tightly regulated through two principal pathways by a number of regulatory and effector molecules.
n proliferating cells, the cell cycle consists of four phases. Gap 1 (G1) is the interval between mitosis and DNA replication that is characterized by cell growth. Replication of DNA occurs during the synthesis (S) phase, which is followed by a second gap phase (G2) during which growth and preparation for cell division occurs. Together, these three stages comprise the interphase phase of the cell cycle. Interphase is followed by the mitotic (M) phase.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico