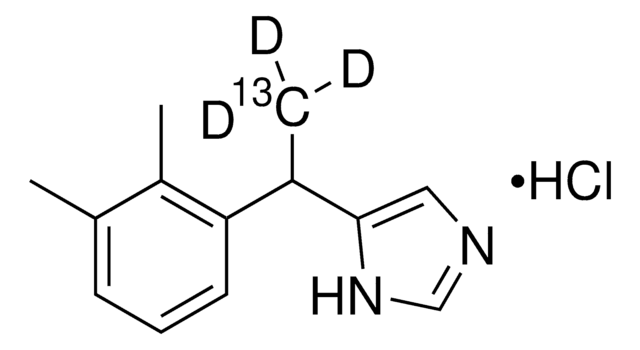
A9611
Atipamezole
≥98% (HPLC)
Sinónimos:
4-(2-Ethyl-2,3-dihydro-1H-inden-2-yl)-1H-Imidazole, Antisedan, MPV 1248
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C14H16N2
Número de CAS:
Peso molecular:
212.29
Número MDL:
Código UNSPSC:
12352200
ID de la sustancia en PubChem:
NACRES:
NA.77
Productos recomendados
Nivel de calidad
Ensayo
≥98% (HPLC)
Formulario
powder
color
white to brown
solubilidad
DMSO: ≥30 mg/mL
temp. de almacenamiento
room temp
cadena SMILES
CCC1(Cc2ccccc2C1)c3c[nH]cn3
InChI
1S/C14H16N2/c1-2-14(13-9-15-10-16-13)7-11-5-3-4-6-12(11)8-14/h3-6,9-10H,2,7-8H2,1H3,(H,15,16)
Clave InChI
HSWPZIDYAHLZDD-UHFFFAOYSA-N
Descripción general
Atipamezole has an imidazole structure and gets localized in the central nervous system on administration.
Aplicación
Atipamezole has been used as a α2-adrenoceptor antagonist in mesencephalic trigeminal nucleus (MTN) neurons, CD4+ T-lymphocyte and human embryonic kidney (HEK293) membrane preparation.
Acciones bioquímicas o fisiológicas
Atipamezole elicits affinity towards adrenoreceptor subtypes namely α2A, α2B and α2C. High levels of atipamezole impairs cognitive functions. It also reverses the adrenoreceptor agonist functionalities. Atipamezole shows no affinity towards muscarinic and dopamine or neurotransmitter receptors. Atipamezole when used along with morphine elicits antinociceptive effects.
Atipamezole is a selective α2 adrenergic blocker. Atipamezole is more potent than yohimbine; it is very selective for α2 adrenergic vs α1 sites, but not selelctive for α2 subtypes.
Atipamezole is a selective α2 adrenergic blocker; neutral antagonist
Características y beneficios
This compound is a featured product for Neuroscience research. Click here to discover more featured Neuroscience products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the α2-Adrenoceptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Tuomas O Lilius et al.
Anesthesia and analgesia, 114(6), 1353-1358 (2012-05-05)
Opioid analgesics are effective in the treatment of chronic pain, but they have serious adverse effects such as development of tolerance and dependence. Adrenergic α(2) agonists and μ-opioid receptor agonists show synergistic potentiation and cross-tolerance in spinal analgesia, whereas α(2)-adrenergic
D Van Vynckt et al.
The Journal of small animal practice, 52(12), 638-644 (2011-10-25)
To assess the influence of two sedation protocols on the degree of lameness in dogs. Fifty lame dogs were allocated to one of two sedation protocols. Group ACPM (acepromazine + methadone; n=25) was sedated with acepromazine and methadone. Group MED
Hong Wei et al.
Basic & clinical pharmacology & toxicology, 112(2), 90-95 (2012-08-21)
Pontine A5, A6 (locus coeruleus) and A7 cell groups provide noradrenergic innervation of the spinal cord. Here, we assessed whether activation of α(2) -adrenoceptors in A7 influences peripheral nerve injury-induced hypersensitivity in the rat, and whether spinal α(2) -adrenoceptors mediate
Michele Barletta et al.
Journal of the American Veterinary Medical Association, 238(9), 1159-1167 (2011-05-03)
To compare efficacy and cardiorespiratory effects of dexmedetomidine and ketamine in combination with butorphanol, hydromorphone, or buprenorphine (with or without reversal by atipamezole) in dogs undergoing castration. Prospective, randomized, split-plot, blinded study. 30 healthy client-owned sexually intact male dogs. Dogs
Andrew P Woolnough et al.
Journal of wildlife diseases, 48(2), 435-443 (2012-04-12)
The Judas technique is a method used for landscape control of feral donkeys (Equus asinus) in northern Australia. Central to the success of any Judas program is the safe, efficient, and humane attachment of the telemetry device. For feral donkeys
Artículos
α2-Adrenoceptors
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico