A5763
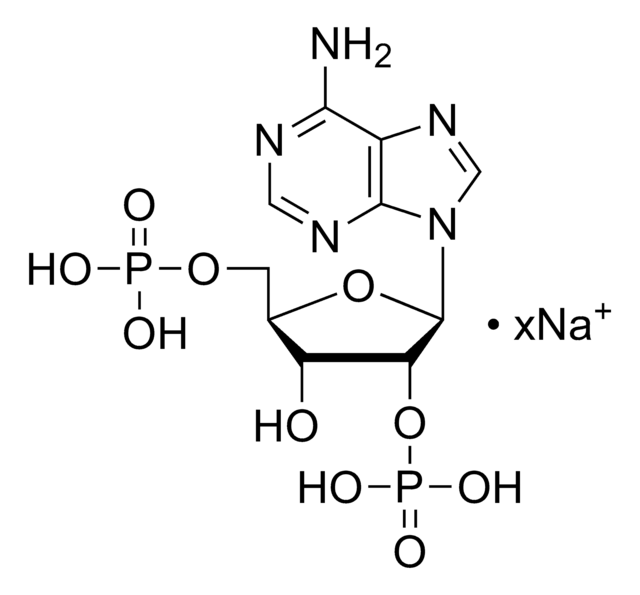
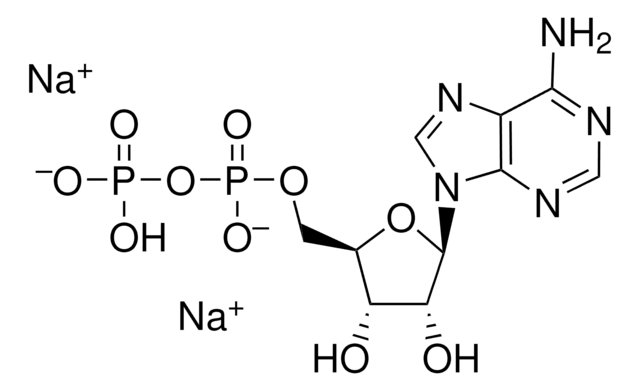
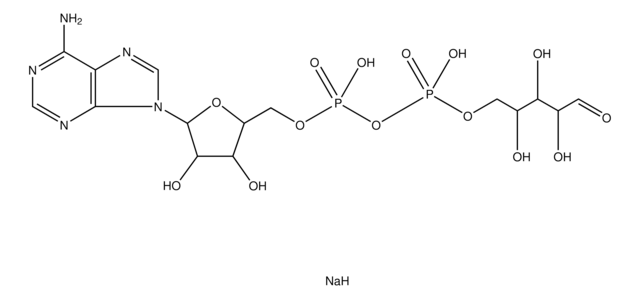
Adenosine 3′,5′-diphosphate disodium salt
≥96%
Sinónimos:
3′-phosphoadenosine 5′-phosphate, 3′-phosphorylated nucleotide, PAP, 3′-Phosphoadenosine 5′-phosphate
About This Item
Productos recomendados
origen biológico
synthetic (inorganic)
Nivel de calidad
Ensayo
≥96%
Formulario
powder
solubilidad
water: 25 mg/mL, clear, colorless to very faintly yellow
temp. de almacenamiento
−20°C
cadena SMILES
[Na].Nc1ncnc2n(cnc12)C3OC(COP(O)(O)=O)C(OP(O)(O)=O)C3O
InChI
1S/C10H15N5O10P2.Na.H/c11-8-5-9(13-2-12-8)15(3-14-5)10-6(16)7(25-27(20,21)22)4(24-10)1-23-26(17,18)19;;/h2-4,6-7,10,16H,1H2,(H2,11,12,13)(H2,17,18,19)(H2,20,21,22);;
Clave InChI
ISROZYFZEAVMSP-UHFFFAOYSA-N
Categorías relacionadas
Descripción general
Aplicación
- to spot sample on cellulose high-performance thin-layer chromatography (HPTLC) plates in two-dimensional thin layer chromatography
- in enzyme activity assay to study the activities of HOS2/FIERY1 wild type
- hos2 mutant and fiery1?2 mutant protein against 3′-phosphoadenosine 5′-phosphate (PAP)
- as a standard for the quantification of phosphoadenosines
Acciones bioquímicas o fisiológicas
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico