A0760
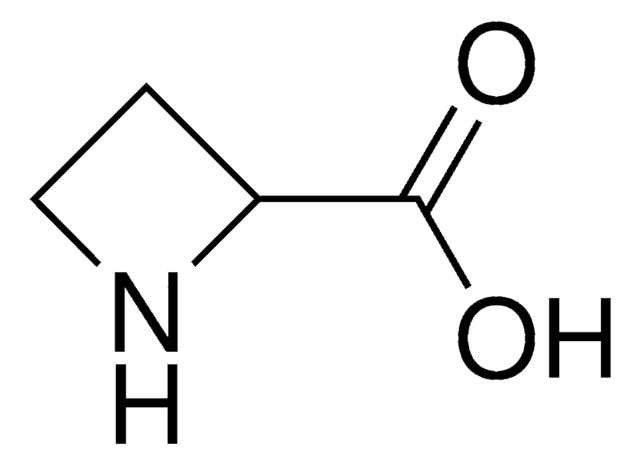
L-Azetidine-2-carboxylic acid
≥99%
Sinónimos:
(S)-Azetidine-2-carboxylic acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C4H7NO2
Número de CAS:
Peso molecular:
101.10
Beilstein:
80680
Número CE:
Número MDL:
Código UNSPSC:
12352209
ID de la sustancia en PubChem:
NACRES:
NA.32
Productos recomendados
Nivel de calidad
Ensayo
≥99%
Formulario
powder
cadena SMILES
OC(=O)[C@@H]1CCN1
InChI
1S/C4H7NO2/c6-4(7)3-1-2-5-3/h3,5H,1-2H2,(H,6,7)/t3-/m0/s1
Clave InChI
IADUEWIQBXOCDZ-VKHMYHEASA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
L-Azetidine-2-carboxylic acid is a non-protein amino acid and teratogenic agent. It is toxic in nature.
Aplicación
L-Azetidine-2-carboxylic acid has been used as a:
- collagen synthesis inhibitor
- protein folding antagonist
- as a standard in liquid chromatography-mass spectrometry
Acciones bioquímicas o fisiológicas
Azetidine-2-carboxylic acid (AZC) triggers protein aggregation or upregulates the expression of an aggregation-prone mutant protein, upon interference with nascent protein folding.
L-Azetidine-2-carboxylic acid is an inhibitor of collagen synthesis that is anti-angiogenic.
L-Azetidine-2-carboxylic acid is an inhibitor of collagen synthesis that is anti-angiogenic. It is a four-membered ring analog of L-proline that causes protein misconstruction when incorporated instead of proline.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Asmita Ghosh et al.
Cellular and molecular life sciences : CMLS, 76(8), 1605-1621 (2019-01-27)
The proteostasis network (PN) comprises a plethora of proteins that are dedicated to aid in protein folding and maintenance; some with overlapping functions. Despite this, there are multiple pathophysiological states associated with depletion of chaperones. This is counter-intuitive, assuming cells
Kate Samardzic et al.
Amino acids, 51(8), 1221-1232 (2019-07-16)
In addition to the 20 protein amino acids that are vital to human health, hundreds of naturally occurring amino acids, known as non-proteinogenic amino acids (NPAAs), exist and can enter the human food chain. Some NPAAs are toxic through their
Azetidine-2-carboxylic acid in garden beets (Beta vulgaris)
Rubenstein E, et al.
Phytochemistry, 67(9), 898-903 (2006)
Essential function of Mec1, the budding yeast ATM/ATR checkpoint-response kinase, in protein homeostasis
Corcoles-Saez I, et al.
Developmental Cell, 46(4), 495-503 (2018)
Nadinath B Nillegoda et al.
Molecular biology of the cell, 21(13), 2102-2116 (2010-05-14)
Quality control systems facilitate polypeptide folding and degradation to maintain protein homeostasis. Molecular chaperones promote folding, whereas the ubiquitin/proteasome system mediates degradation. We show here that Saccharomyces cerevisiae Ubr1 and Ubr2 ubiquitin ligases promote degradation of unfolded or misfolded cytosolic
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico