654380
Tunicamycin
from Strepomyces lysosuperficus, ≥98% (A+B+C+D, HPLC), powder, N-acetylglucosamine transferase, Calbiochem
Sinónimos:
Tunicamycin, Streptomyces lysosuperficus
About This Item
Productos recomendados
Nombre del producto
Tunicamycin, Streptomyces lysosuperficus,
descripción
Tunicamycin, Streptomyces lysosuperficus
Nivel de calidad
Ensayo
≥98% (A+B+C+D, HPLC)
Formulario
powder
fabricante / nombre comercial
Calbiochem®
solubilidad
DMSO: 10 mg/mL
DMF: soluble
pyridine: soluble
Condiciones de envío
ambient
temp. de almacenamiento
2-8°C
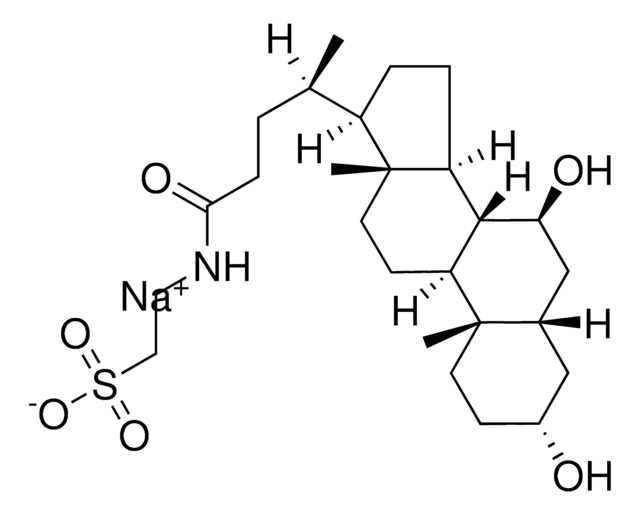
cadena SMILES
N4(C=CC(=O)NC4=O)[C@@H]1O[C@@H]([C@H]([C@H]1O)O)C(O)C[C@H]2O[C@@H]([C@@H]([C@H]([C@H]2O)O)NC(=O)\C=C\CC(C)C)O[C@H]3O[C@@H]([C@H]([C@@H]([C@H]3NC(=O)C)O)O)CO
InChI
1S/C30H46N4O16/c1-11(2)5-4-6-16(38)32-19-23(43)20(40)14(47-29(19)50-28-18(31-12(3)36)22(42)21(41)15(10-35)48-28)9-13(37)26-24(44)25(45)27(49-26)34-8-7-17(39)33-30(34)46/h4,6-8,11,13-15,18-29,35,37,40-45H,5,9-10H2,1-3H3,(H,31,36)(H,32,38)(H,33,39,46)/b6-4+/t13?,14-,15-,18-,19-,20+,21-,22-,23-,24+,25-,26-,27-,28-,29-/m1/s1
Clave InChI
ZHSGGJXRNHWHRS-PEALBESXSA-N
Descripción general
Acciones bioquímicas o fisiológicas
N-linked glycosylation
thrombin-induced Ca2+ mobilization in cells
Advertencia
Nota de preparación
Reconstitución
Otras notas
Price, B.D., et al. 1992. J. Cell Physiol.152, 545.
Información legal
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 1 Oral
Código de clase de almacenamiento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico




![PERK Inhibitor I, GSK2606414 GSK2606414 is a cell-permeable, highly potent inhibitor of EIF2AK3/PERK (IC₅₀ = 0.4 nM; [ATP] = 5 µM). Targets PERK in its inactive DFG conformation at the ATP-binding region.](/deepweb/assets/sigmaaldrich/product/structures/180/559/efa716dc-d5fe-4339-a6f0-0103084fc04a/640/efa716dc-d5fe-4339-a6f0-0103084fc04a.png)