77634
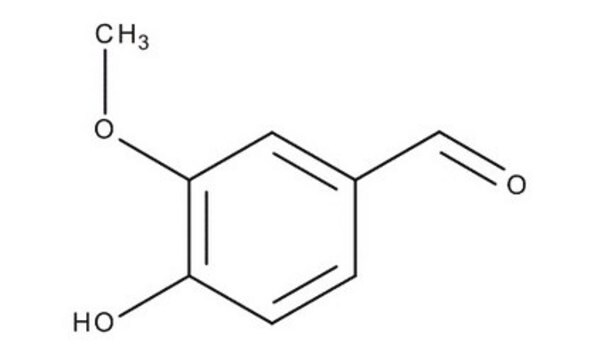
Vainillina
traceable to primary standards (LGC)
Sinónimos:
4-Hidroxi-3-metoxibenzaldehído, Aldehído vanílico
About This Item
Productos recomendados
grado
analytical standard
Nivel de calidad
densidad de vapor
5.3 (vs air)
presión de vapor
>0.01 mmHg ( 25 °C)
calidad
traceable to primary standards (LGC)
caducidad
limited shelf life, expiry date on the label
bp
170 °C/15 mmHg (lit.)
mp
81-83 °C (lit.)
aplicaciones
food and beverages
pharmaceutical
Formato
neat
cadena SMILES
COc1cc(C=O)ccc1O
InChI
1S/C8H8O3/c1-11-8-4-6(5-9)2-3-7(8)10/h2-5,10H,1H3
Clave InChI
MWOOGOJBHIARFG-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Mettler-Toledo calibration substance ME 51143093, vanillin is an analytical standard for use in the regular checking of Mettler-Toledo melting point instrument. Its value equals an average of 6 to 12 measurements with a Mettler-Toledo MP90 Excellence instrument that is calibrated against primary standards. The melting point is validated by Capillary method according to European Pharmacopeia (2.2.14.)
Aplicación
Características y beneficios
- Traceable to a primary standard (LGC, London)
- Melting point evaluation conducted in both thermodynamic and pharmacopeia modes for physically correct and heating rate dependent melting point determinations, respectively
- Provided with certificates of analysis and safety data sheet
- A product of analytical standard grade to help meet the QC/QA requirements of melting point determination
- Standard deviation up to ± 0.2 °C
Productos recomendados
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
319.6 - 321.4 °F - closed cup
Punto de inflamabilidad (°C)
159.8 - 160.8 °C - closed cup
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico