03359123001
Roche
Alkaline Phosphatase recombinant, highly active
EIA Grade, from Pichia Pastoris
About This Item
Productos recomendados
Nivel de calidad
Análisis
≥95% (HPLC)
formulario
buffered aqueous solution (ready-to-use, 20 mg/ml)
actividad específica
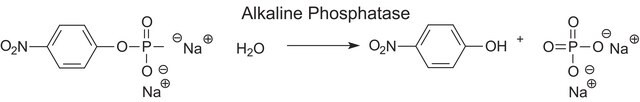
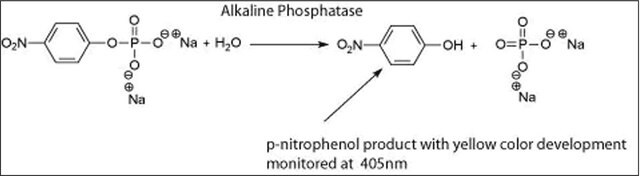
≥7000 units/mg protein (At 37 °C with pNPP and DEA as the substrates)
envase
pkg of 10 mg (500 μl)
fabricante / nombre comercial
Roche
concentración
20 mg/mL (ready-to-use)
pH óptimo
8.0(Stability)
9.8(Activity)
Condiciones de envío
wet ice
temp. de almacenamiento
2-8°C
Descripción general
The recombinant enzyme guarantees superior lot-to-lot consistency and completely eliminates the risk of BSE.
Especificidad
Aplicación
Acciones bioquímicas o fisiológicas
Especificaciones
Forma física
Nota de preparación
Otras notas
Código de clase de almacenamiento
12 - Non Combustible Liquids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
No data available
Punto de inflamabilidad (°C)
No data available
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico