TZHVDV205
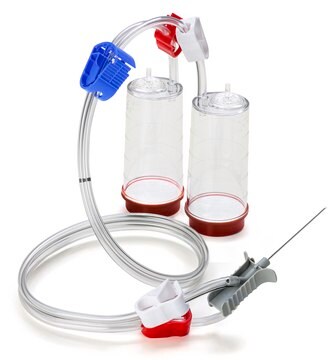
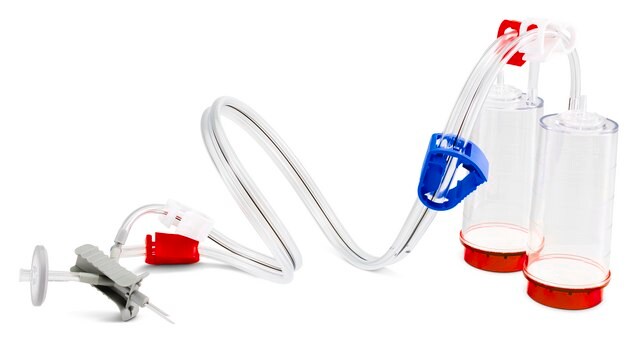
Steritest® NEO Device
For soluble powders in vials with septa. Red base canister with vented double needle for small vials with septa. Double packed.
Sinónimos:
Red Base Steritest® NEO device for sterility testing, Sterility testing device, membrane filtration device, membrane filtration canister, closed membrane filtration
About This Item
Productos recomendados
Materiales
Nylon 66 adapter (for needle)
PVC tubing (double lumen)
PVDF membrane
plain filter
stainless steel (for needle)
styrene-acrylonitrile (SAN) (for canister)
Nivel de calidad
Agency
EP (2.6.1)
JP (4.06)
USP 71
esterilidad
sterile; γ-irradiated
fabricante / nombre comercial
Steritest®
envase
pkg of 10 blisters in 2 bags of 5 blisters per box, Double packed
Parámetros
120 mL sample volume (graduation marks at 25, 50, 75 and 100 mL)
3.1 bar max. inlet pressure (45 psi) at 25 °C
45 °C max. temp.
L tubo
850 mm
color
red Canister Base
matriz
Durapore®
tamaño de poro
0.45 μm pore size
entrada
sample type pharmaceutical(s)
aplicaciones
pharmaceutical
sterility testing
Condiciones de envío
ambient
Descripción general
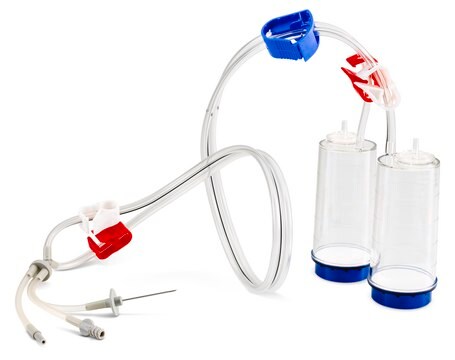
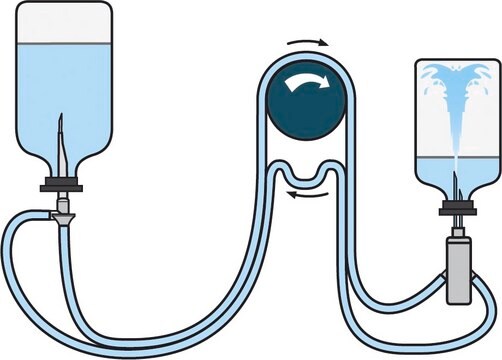
Steritest® NEO is a membrane filtration device for sterility testing of filterable pharmaceutical products. The device simplifies every aspect of testing, from handling to traceability. The closed system ensures that pharmaceutical products are never exposed to the environment, minimizes false positives, and offers the highest levels of quality & reliability. This test system offers an optimized and fully regulatory compliant testing process when used with the Steritest® Symbio pump, specific accessories and high-quality culture media and rinsing fluids. The device is gamma sterilized and double packed for the quick transfer into sterility testing environments, simplifying decontamination procedures and saving time. The device simultaneously dissolves/dilutes the sample in sterile diluent and transfers the resulting solution to canisters. A small diameter double needle adapter is used for small vials with septum. The red canister base indicates low absorption. Durapore® hydrophilic Poly vinylidene fluoride (PVDF) membrane and specific drain design. This optimizes the rinsing of products that inhibit microbial growth.
Aplicación
Características y beneficios
- One-stop-shop for sterility testing with our devices, pumps, media, fluids, and services
- Steritest® devices are manufactured in our Center of Excellence in Molsheim, France, with high-quality control standards maintaining the Certificate of Quality for each lot.
- New needle design
- Smarter workflow
- Completely closed set up
- Consistent performance
- New tubing disconnection tool
Envase
Información legal
configurado para
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico