W318101
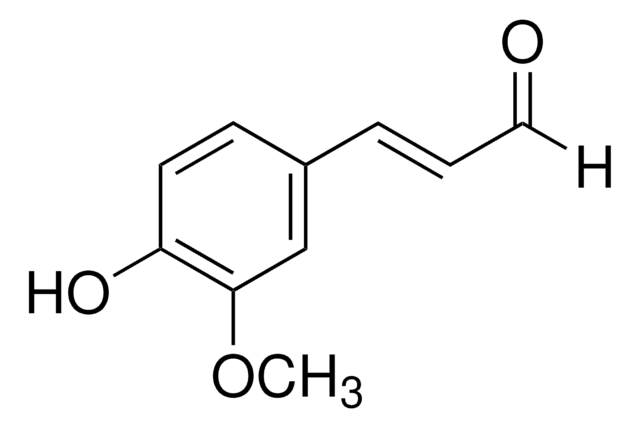
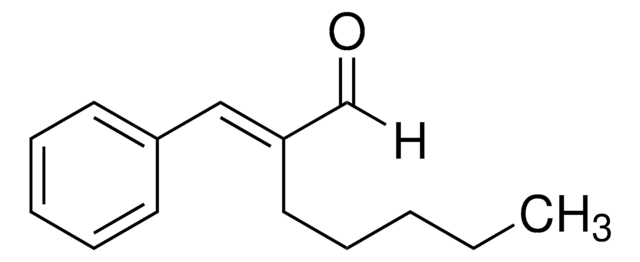
2-Methoxycinnamaldehyde
natural, 98%, FG
Sinónimos:
o-Methoxycinnamaldehyde
About This Item
Fragrance grade
Halal
Kosher
natural
Productos recomendados
grado
FG
Fragrance grade
Halal
Kosher
natural
Nivel de calidad
Agency
follows IFRA guidelines
cumplimiento norm.
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 172.515
Ensayo
98%
bp
160-161 °C/12 mmHg (lit.)
mp
44.0-49.0 °C (lit.)
aplicaciones
flavors and fragrances
Documentación
see Safety & Documentation for available documents
alérgeno alimentario
no known allergens
alérgeno de la fragancia
no known allergens
Organoléptico
cinnamon; woody; spicy; sweet
cadena SMILES
[H]C(=O)C=Cc1ccccc1OC
InChI
1S/C10H10O2/c1-12-10-7-3-2-5-9(10)6-4-8-11/h2-8H,1H3/b6-4+
Clave InChI
KKVZAVRSVHUSPL-GQCTYLIASA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Aplicación
- Network pharmacology combined with molecular docking and experimental validation to explore the potential mechanism of Cinnamomi ramulus against ankylosing spondylitis.: This study investigates the anti-inflammatory potential of 2-Methoxycinnamaldehyde in Cinnamomi ramulus. Its application extends to novel therapeutic approaches for treating ankylosing spondylitis, demonstrating significant implications for medicinal chemistry and pharmacology (Wei et al., 2023).
- Ramulus Cinnamomi essential oil exerts an anti-inflammatory effect on RAW264.7 cells through N-acylethanolamine acid amidase inhibition.: The study elaborates on the anti-inflammatory activities of 2-Methoxycinnamaldehyde, offering a biochemical pathway that could be exploited in anti-inflammatory drug design (Jia et al., 2023).
- Metabolomics-Driven Exploration of the Antibacterial Activity and Mechanism of 2-Methoxycinnamaldehyde.: This article offers insights into the antibacterial properties of 2-Methoxycinnamaldehyde, using metabolomics to explore its mechanism of action, significant for developments in antimicrobial treatments (Qian et al., 2022).
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
235.4 °F
Punto de inflamabilidad (°C)
113 °C
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico