804088
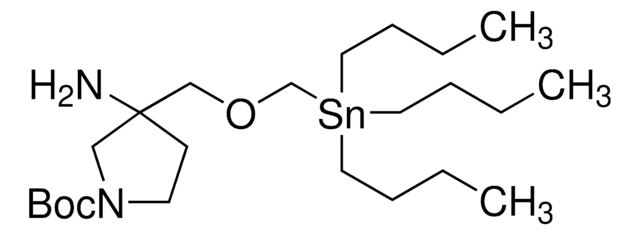
SnAP 2-Spiro-(4-Pip) M Reagent
Sinónimos:
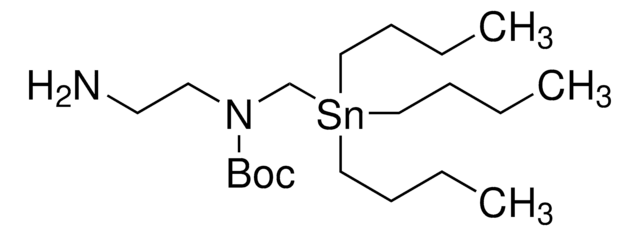
tert-butyl 4-(aminomethyl)-4-((tributylstannyl)methoxy)piperidine-1-carboxylate
About This Item
Productos recomendados
Formulario
liquid
índice de refracción
n/D 1.492
densidad
1.146
grupo funcional
amine
ether
temp. de almacenamiento
−20°C
cadena SMILES
CCCC[Sn](CCCC)(COC1(CCN(C(OC(C)(C)C)=O)CC1)CN)CCCC
InChI
1S/C12H23N2O3.3C4H9.Sn/c1-11(2,3)17-10(15)14-7-5-12(9-13,16-4)6-8-14;3*1-3-4-2;/h4-9,13H2,1-3H3;3*1,3-4H2,2H3;
Clave InChI
LEBKPKYDLQUOJO-UHFFFAOYSA-N
Descripción general
Aplicación
Automate your N-heterocycle formation with Synple Automated Synthesis Platform (SYNPLE-SC002)
Otras notas
Professor product portal: Jeffrey Bode Research Group
SnAP Reagents for the Synthesis of Piperazines and Morpholines
SnAP reagents for the one-step synthesis of medium-ring saturated N-heterocycles from aldehydes
SnAP Reagents for a Cross-Coupling Approach to the One-Step Synthesis of Saturated N-Heterocycles
Bespoke SnAP Reagents for the Synthesis of C-Substituted Spirocyclic and Bicyclic Saturated N-Heterocycles
Producto relacionado
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2 - STOT RE 1
Código de clase de almacenamiento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
>230.0 °F
Punto de inflamabilidad (°C)
> 110 °C
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lo sentimos, en este momento no disponemos de COAs para este producto en línea.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Protocolos
Saturated N-heterocyclic building blocks or SnAP Reagents are of growing importance for the convenient synthesis of medium-ring saturated N-heterocycles, including bicyclic and spirocyclic structures. SnAP reagents are stable and readily available and can be coupled with widely available aromatic, heteroaromatic, aliphatic, and glyoxylic aldehydes.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico