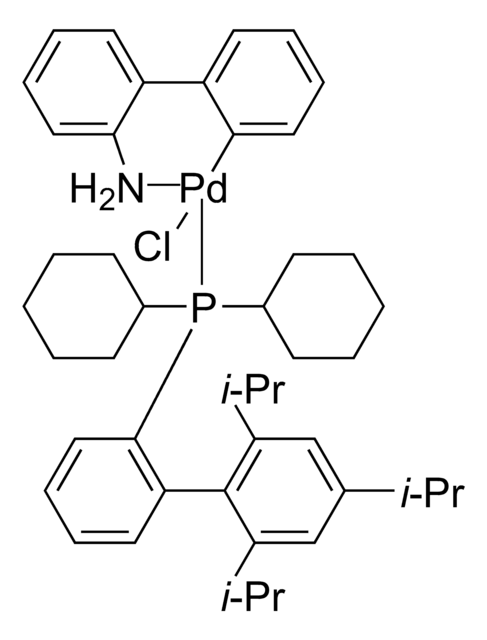
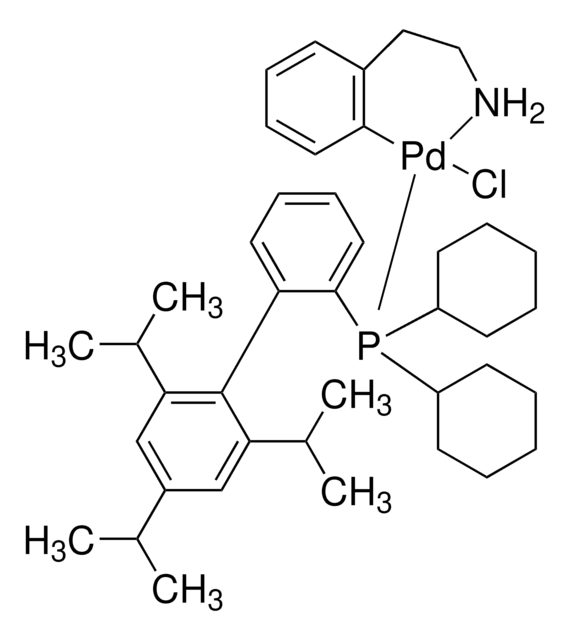
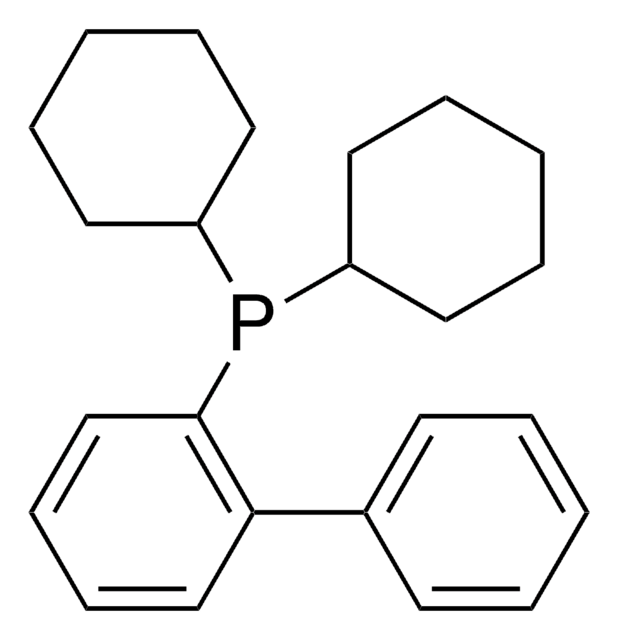
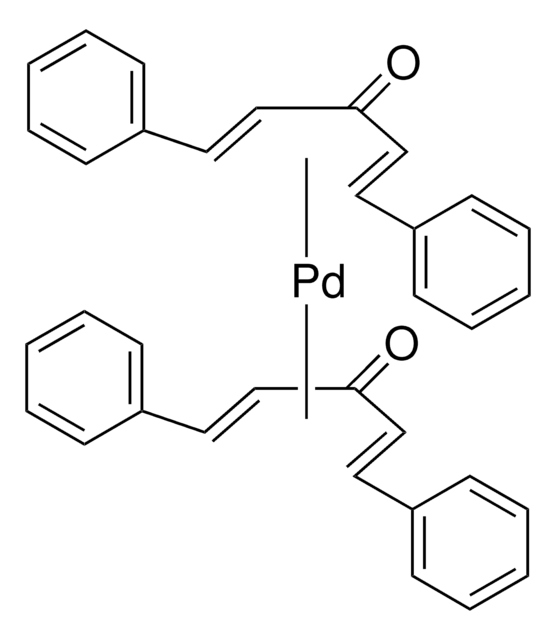
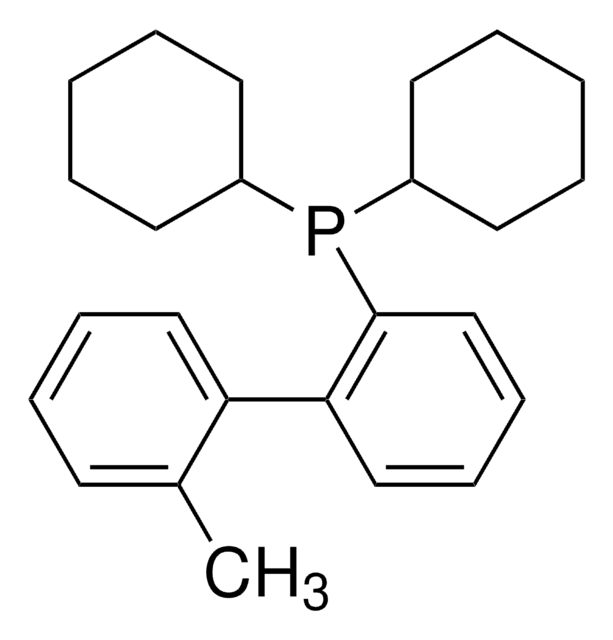
638064
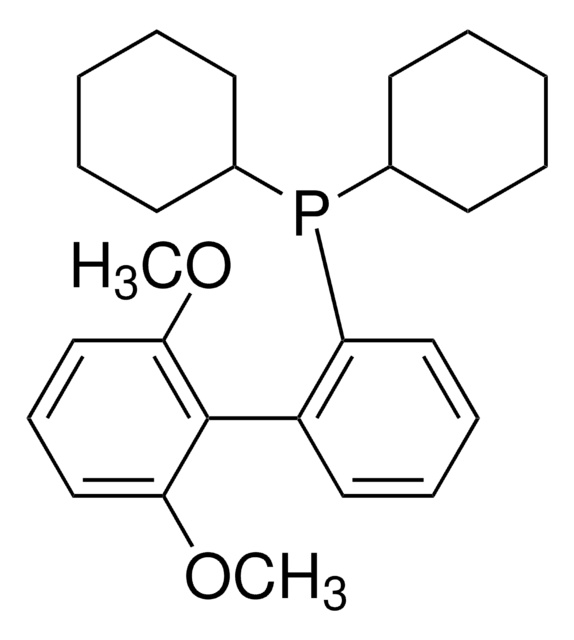
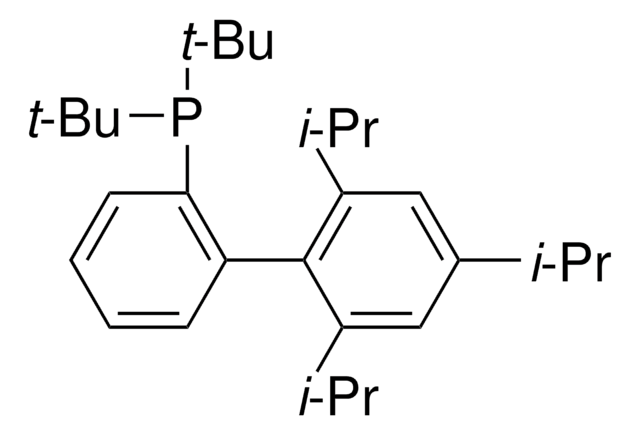
XPhos
98%
Sinónimos:
2-Dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl
About This Item
Productos recomendados
Nivel de calidad
Ensayo
98%
idoneidad de la reacción
reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-C Bond Formation
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Hiyama Coupling
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Stille Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
puntuación de productos alternativos más sostenibles
old score: 2
new score: 1
Find out more about DOZN™ Scoring
características de los productos alternativos más sostenibles
Waste Prevention
Atom Economy
Use of Renewable Feedstocks
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
187-190 °C (lit.)
grupo funcional
phosphine
categoría alternativa más sostenible
cadena SMILES
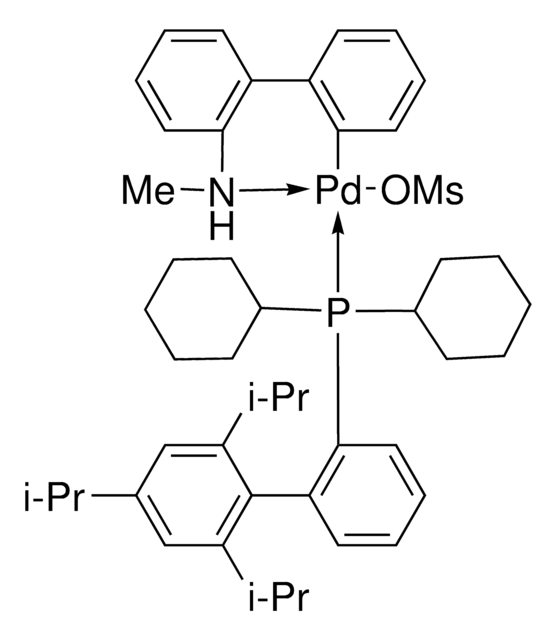
CC(C)C1=CC(C(C)C)=CC(C(C)C)=C1C2=C(P(C3CCCCC3)C4CCCCC4)C=CC=C2
InChI
1S/C33H49P/c1-23(2)26-21-30(24(3)4)33(31(22-26)25(5)6)29-19-13-14-20-32(29)34(27-15-9-7-10-16-27)28-17-11-8-12-18-28/h13-14,19-25,27-28H,7-12,15-18H2,1-6H3
Clave InChI
UGOMMVLRQDMAQQ-UHFFFAOYSA-N
Descripción general
Aplicación
Direct annulation of 2-haloanilines to indoles and tryptophans catalyzed by Pd. Synthesis of regioregular polythiophenes.
For small scale and high throughput uses, product is also available as ChemBeads (928364)
On the Way Towards Greener Transition-Metal-Catalyzed Processes as Quantified by E Factors
- Preparation of functionalized benzylic sulfones via palladium-catalyzed Negishi cross-coupling between alkyl sulfones and aryl halides.
- Along with pre-milled palladium(II) acetate as a pre-catalyst for the Stille cross-coupling of aryl chlorides with tributylarylstannanes to form the corresponding biaryl compounds.
- Along with platinum chloride to catalyze the hydrosilylation of terminal arylalkynes with silanes to form functionalized β-(E)-vinylsilanes.
Información legal
Producto relacionado
Código de clase de almacenamiento
13 - Non Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
A variety of palladium-catalyzed cross-coupling reactions can be run under mild room temperature conditions in water with TPGS- 750-M, using a variety of commercially available palladium complexes and ligands.
Buchwald Phosphine Ligands
TPGS-750-M, a second generation surfactant, is useful for room temperature, palladium and ruthenium-catalyzed reactions in water. Reactions include the Heck reaction, Suzuki-Miyaura reaction, Sonogashira reaction, Buchwald-Hartwig amination reaction, Negishi reaction, and olefin metathesis.
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico