634492
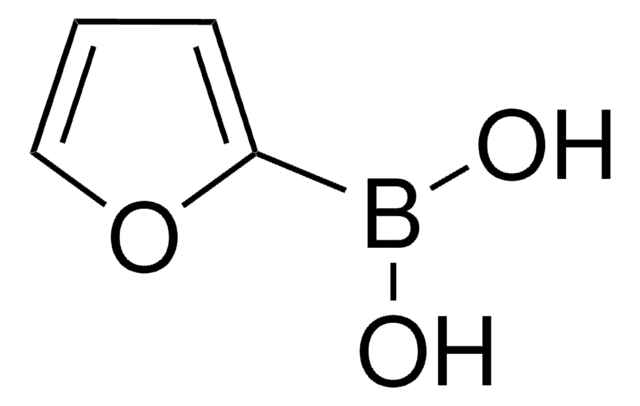
4-Pyridinylboronic acid
90%
Sinónimos:
4-Pyridineboronic acid, 4-Pyridylboronic acid
About This Item
Productos recomendados
Nivel de calidad
Análisis
90%
formulario
solid
mp
>300 °C (lit.)
temp. de almacenamiento
−20°C
cadena SMILES
OB(O)c1ccncc1
InChI
1S/C5H6BNO2/c8-6(9)5-1-3-7-4-2-5/h1-4,8-9H
Clave InChI
QLULGIRFKAWHOJ-UHFFFAOYSA-N
Categorías relacionadas
Descripción general
Aplicación
- Palladium-catalyzed Suzuki-Miyaura coupling reactions
- Ligand-free palladium-catalyzed Suzuki coupling reaction under microwave irradation
Reagent used in Preparation of
- HIV-1 protease inhibitors
- Potential cancer threapeutics, such as PDK1 and protein kinase CK2 inhibitors
Otras notas
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
This brochure contains a comprehensive selection of boronic acids, boronic acid esters, diboron esters, and transition-metal catalysts useful for the Suzuki–Miyaura coupling reaction
The Suzuki-Miyaura cross-coupling reaction is an important and extensively used reaction in organic chemistry with applications in polymer science and in the fine chemicals and pharmaceutical industries.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico