328367
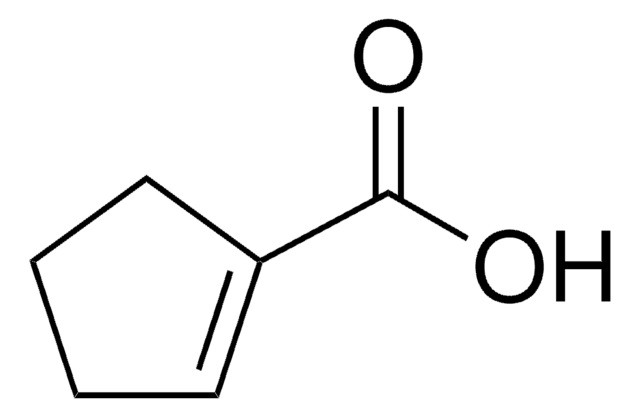
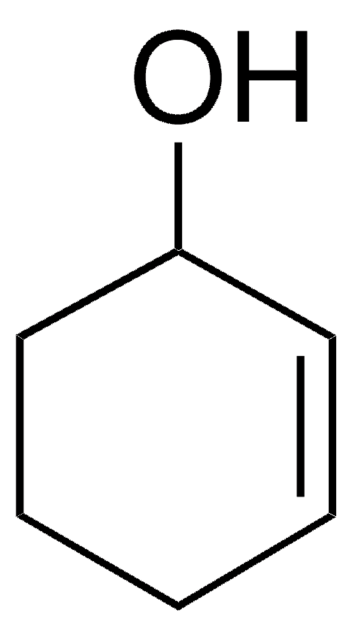
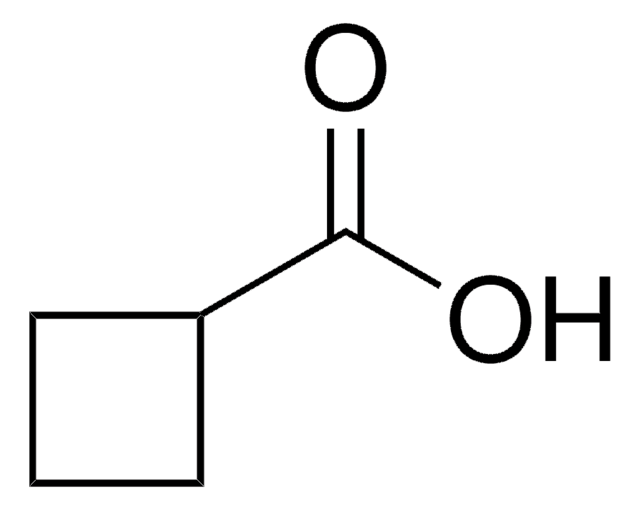
1-Cyclohexene-1-carboxylic acid
97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C6H9CO2H
Número de CAS:
Peso molecular:
126.15
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
97%
Formulario
solid
bp
133-135 °C/14 mmHg (lit.)
mp
35-39 °C (lit.)
densidad
1.101 g/mL at 25 °C (lit.)
grupo funcional
carboxylic acid
cadena SMILES
OC(=O)C1=CCCCC1
InChI
1S/C7H10O2/c8-7(9)6-4-2-1-3-5-6/h4H,1-3,5H2,(H,8,9)
Clave InChI
NMEZJSDUZQOPFE-UHFFFAOYSA-N
Categorías relacionadas
Descripción general
1-Cyclohexene-1-carboxylic acid was identified as intermediate during the anaerobic decomposition of benzoic acid by a methanogenic consortium.
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Dam. 1 - Skin Corr. 1B
Código de clase de almacenamiento
8A - Combustible corrosive hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
235.4 °F - closed cup
Punto de inflamabilidad (°C)
113 °C - closed cup
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
C L Keith et al.
Archives of microbiology, 118(2), 173-176 (1978-08-01)
A possible pathway for the anaerobic utilization of benzoic acid by a methanogenic consortium is suggested. Cyclohexane carboxylic acid and 1-cyclohexene-1-carboxylic acid have been identified as intermediates before ring rupture. Suprisingly, 3-cyclohexene-1-carboxylic acid interferes with utilization of other cyclic acids.
Mariana G de Oliveira et al.
American journal of physiology. Renal physiology, 315(3), F460-F468 (2018-05-03)
Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) is a chronic inflammatory disease without consistently effective treatment. We investigate the role of toll-like receptor 4 (TLR4) on voiding dysfunction and inflammation in the cyclophosphamide (CYP)-induced mouse cystitis. Male C57BL/6 [wild-type, (WT)] and/or TLR4
K A Reynolds et al.
Journal of bacteriology, 174(12), 3850-3854 (1992-06-01)
A novel NADPH-dependent enoyl reductase, catalyzing the conversion of 1-cyclohexenylcarbonyl coenzyme A (1-cyclohexenylcarbonyl-CoA) to cyclohexylcarbonyl-CoA, was purified to homogeneity from Streptomyces collinus. This enzyme, a dimer with subunits of identical M(r) (36,000), exhibits a Km of 1.5 +/- 0.3 microM
Xin Xie et al.
Carbohydrate polymers, 225, 115223-115223 (2019-09-16)
A polysaccharide isolated from Strongylocentrotus nudus eggs (SEP) reportedly displays immune activity in vivo. Here, its effect and underlying mechanism in the treatment of pancreatic cancer were investigated. SEP obviously inhibited pancreatic cancer growth by activating NK cells in vitro/vivo
M S Elshahed et al.
Applied and environmental microbiology, 67(4), 1728-1738 (2001-04-03)
The metabolism of benzoate, cyclohex-1-ene carboxylate, and cyclohexane carboxylate by "Syntrophus aciditrophicus" in cocultures with hydrogen-using microorganisms was studied. Cyclohexane carboxylate, cyclohex-1-ene carboxylate, pimelate, and glutarate (or their coenzyme A [CoA] derivatives) transiently accumulated during growth with benzoate. Identification was
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico