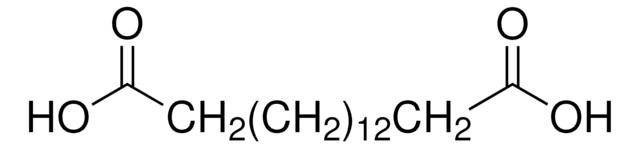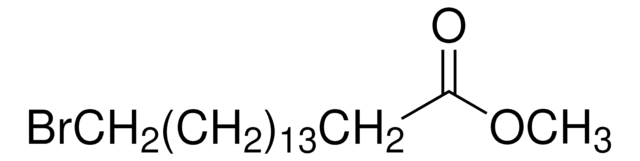177490
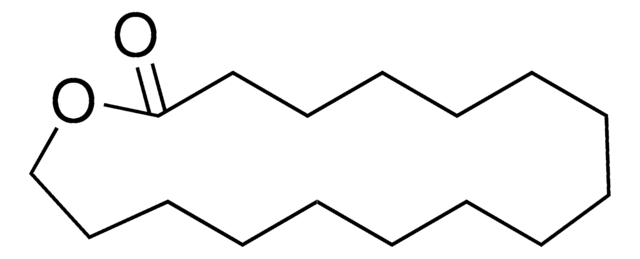
16-Hydroxyhexadecanoic acid
98%
Sinónimos:
16-Hydroxypalmitic acid, Juniperic acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
HO(CH2)15CO2H
Número de CAS:
Peso molecular:
272.42
Beilstein:
1783998
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
98%
Formulario
solid
mp
94-98 °C (lit.)
grupo funcional
carboxylic acid
hydroxyl
cadena SMILES
OCCCCCCCCCCCCCCCC(O)=O
InChI
1S/C16H32O3/c17-15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-16(18)19/h17H,1-15H2,(H,18,19)
Clave InChI
UGAGPNKCDRTDHP-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
16-Hydroxyhexadecanoic acid was used in the synthesis of dihydroxypalmitic acids. It was also used to induce the expression of two GRP genes of Arabidopsis thaliana, AtGRP5 and AtGRP23.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Sapa Hima Rani et al.
The Journal of biological chemistry, 285(49), 38337-38347 (2010-10-06)
A key step in the triacylglycerol (TAG) biosynthetic pathway is the final acylation of diacylglycerol (DAG) by DAG acyltransferase. In silico analysis has revealed that the DCR (defective in cuticular ridges) (At5g23940) gene has a typical HX(4)D acyltransferase motif at
Akihisa Abe et al.
Journal of biochemistry, molecular biology, and biophysics : JBMBB : the official journal of the Federation of Asian and Oceanian Biochemists and Molecular Biologists (FAOBMB), 6(1), 37-43 (2002-08-21)
We have found that omega-hydroxy palmitic acid (16-hydroxy palmitic acid, omega-HPA) has both cell growth inhibiting and cell death inducing actions on human lung adenosquamous carcinoma cell line H596 and adenocarcinoma cell line A549. Further, these effects were dose- and
Jong Ho Park et al.
Plant physiology and biochemistry : PPB, 46(11), 1015-1018 (2008-07-29)
Glycine-rich proteins (GRPs) belong to a large family of heterogenous proteins that are enriched in glycine residues. The expression of two GRP genes of Arabidopsis thaliana, AtGRP5 and AtGRP23, was induced by 16-hydroxypalmitic acid (HPA), a major component of cutin.
G D Rees et al.
Biochimica et biophysica acta, 1257(3), 239-248 (1995-08-03)
Five microbial lipases from Chromobacterium viscosum, Candida cylindracea, Pseudomonas (source Fluka), Pseudomonas (source Genzyme) and lipoprotein lipase ex Microbial (Genzyme) have been screened for lactonisation activity towards 16-hydroxyhexadecanoic acid (HHA) in a variety of different w/o microemulsion systems. With the
Jean-Paul Douliez
Journal of colloid and interface science, 271(2), 507-510 (2004-02-20)
The interaction between cutin and suberin monomers, i.e., omega -hydroxylpalmitic acid, alpha, omega -hexadecanedioic acid, alpha, omega --hexadecanediol, 12-hydroxylstearic acid, and phospholipid vesicles biomimicking the lipid structure of plant cell membranes has been studied by optical and transmission electron microscopy
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico