Wichtige Dokumente
C2020
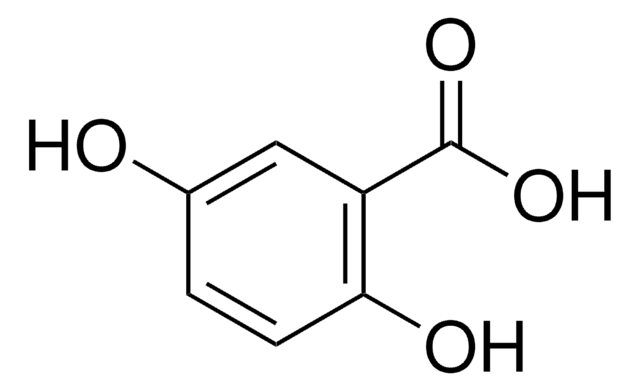
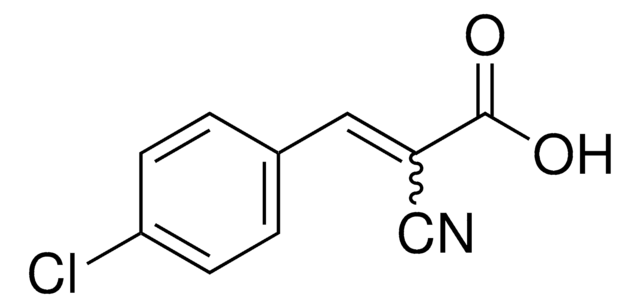
α-Cyano-4-hydroxyzimtsäure
≥98% (TLC), powder, monocarboxylic acid transport inhibitor
Synonym(e):
α-CCA, α-CHCA, α-Cyano, 4-HCCA, ACCA
About This Item
Empfohlene Produkte
Produktbezeichnung
α-Cyano-4-hydroxyzimtsäure, ≥98% (TLC), powder
Qualitätsniveau
Assay
≥98% (TLC)
Form
powder
Farbe
yellow
mp (Schmelzpunkt)
245-250 °C (lit.)
Löslichkeit
H2O: slightly soluble
methanol: water: soluble
polar organic solvents: soluble
Lagertemp.
2-8°C
SMILES String
OC(=O)\C(=C\c1ccc(O)cc1)C#N
InChI
1S/C10H7NO3/c11-6-8(10(13)14)5-7-1-3-9(12)4-2-7/h1-5,12H,(H,13,14)/b8-5+
InChIKey
AFVLVVWMAFSXCK-VMPITWQZSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
Biochem./physiol. Wirkung
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Skin Sens. 1B
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
We presents an article about the Warburg effect, and how it is the enhanced conversion of glucose to lactate observed in tumor cells, even in the presence of normal levels of oxygen. Otto Heinrich Warburg demonstrated in 1924 that cancer cells show an increased dependence on glycolysis to meet their energy needs, regardless of whether they were well-oxygenated or not.
Verwandter Inhalt
DISCOVER Bioactive Small Molecules for Nitric Oxide & Cell Stress Research
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.