B8959
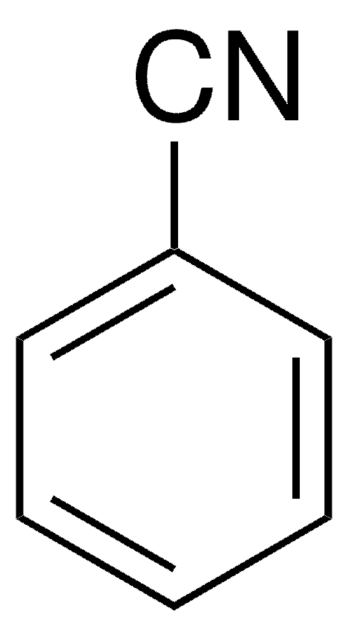
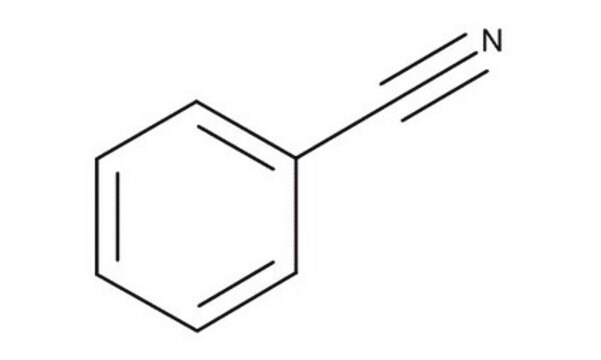
Benzonitril
ReagentPlus®, 99%
Synonym(e):
Phenylcyanid
About This Item
Empfohlene Produkte
Qualitätsniveau
Produktlinie
ReagentPlus®
Assay
99%
Form
liquid
Expl.-Gr.
0.34-6.3 %
Brechungsindex
n20/D 1.528 (lit.)
bp
191 °C (lit.)
mp (Schmelzpunkt)
−13 °C (lit.)
SMILES String
N#Cc1ccccc1
InChI
1S/C7H5N/c8-6-7-4-2-1-3-5-7/h1-5H
InChIKey
JFDZBHWFFUWGJE-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- An electrochemical solvent to investigate the electrochemistry, spectroscopic properties, and reactivity of a series of cobalt porphyrins with various substituents.
- Building block or starting material in various organic synthesis reactions.
- Employed in coupling reactions, such as Suzuki couplings or Heck reactions, to facilitate the formation of carbon-carbon bonds.
Rechtliche Hinweise
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Dermal - Acute Tox. 4 Oral
Lagerklassenschlüssel
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 1
Flammpunkt (°F)
158.0 °F - closed cup
Flammpunkt (°C)
70 °C - closed cup
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.