Wichtige Dokumente
T-058
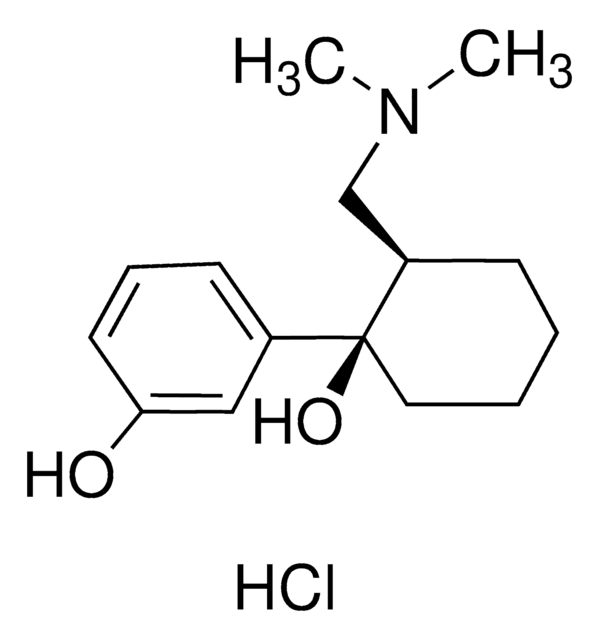
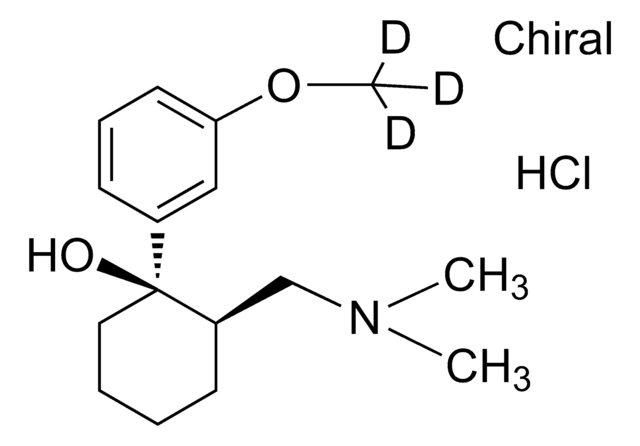
Tapentadol -hydrochlorid -Lösung
1.0 mg/mL in methanol (as free base), ampule of 1 mL, certified reference material, Cerilliant®
Synonym(e):
Tapentadol -hydrochlorid -Lösung
About This Item
Empfohlene Produkte
Qualität
certified reference material
Qualitätsniveau
Form
liquid
Leistungsmerkmale
Snap-N-Spike®/Snap-N-Shoot®
Verpackung
ampule of 1 mL
Hersteller/Markenname
Cerilliant®
drug control
Narcotic Licence Schedule A (Switzerland); estupefaciente (Spain); Decreto Lei 15/93: Tabela IA (Portugal)
Konzentration
1.0 mg/mL in methanol (as free base)
Methode(n)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
Anwendung(en)
forensics and toxicology
Format
single component solution
Lagertemp.
−20°C
SMILES String
Cl.CC[C@H]([C@@H](C)CN(C)C)c1cccc(O)c1
InChI
1S/C14H23NO.ClH/c1-5-14(11(2)10-15(3)4)12-7-6-8-13(16)9-12;/h6-9,11,14,16H,5,10H2,1-4H3;1H/t11-,14+;/m0./s1
InChIKey
ZELFLGGRLLOERW-YECZQDJWSA-N
Angaben zum Gen
human ... OPRM1(4988) , SLC6A2(6530)
Allgemeine Beschreibung
Anwendung
- Formulierung mit verlängerter Wirkstofffreisetzung bei chronischen Schmerzen: Tapentadol-Hydrochlorid-Formulierungen mit verlängerter Wirkstofffreisetzung wurden entwickelt, um eine anhaltende Schmerzlinderung bei chronischen Erkrankungen zu bieten. Diese Formulierung reduziert die Dosierungshäufigkeit und verbessert die Patienten-Compliance durch ein konsistentes Schmerzmanagement (Fara et al., 2017).
- Pharmazeutische Qualitätskontrolle und Forschung: Hochreine Tapentadol-Hydrochlorid-Lösungen sind für die pharmazeutische Forschung und Qualitätskontrolle von entscheidender Bedeutung. Diese Lösungen werden zur Kalibrierung von Analysegeräten und zur Gewährleistung der Konsistenz und Sicherheit pharmazeutischer Produkte verwendet (Fejos et al., 2014).
- Transdermale Abgabesysteme: Im Rahmen innovativer Forschungsarbeiten wurden PEGylierte, ultraverformbare Transferosomen für die transdermale Abgabe von Tapentadol entwickelt, die dessen Bioverfügbarkeit und schmerzlindernde Wirkung verbessern. Diese Methode bietet eine nicht-invasive Alternative zur Schmerzbehandlung (Deng et al., 2022).
- Innovative Lösungen für das Schmerzmanagement: Die Forschung zu neuen Abgabemethoden, wie z. B. die intranasale Verabreichung unter Verwendung von Chitosan-Nanopartikeln, zielt darauf ab, das therapeutische Potenzial und die Patienten-Compliance der Tapentadol-Hydrochlorid-Lösung zur Schmerzbehandlung zu verbessern (Javia & Thakkar, 2017).
Rechtliche Hinweise
Ähnliches Produkt
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Zielorgane
Eyes,Central nervous system
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 2
Flammpunkt (°F)
49.5 °F - closed cup
Flammpunkt (°C)
9.7 °C - closed cup
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Protokolle
-THC solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material
To optimize hydrolysis using β-glucuronidase, factors such as incubation time, temperature, hydrolysis pH, enzyme source, and enzyme concentration must be evaluated for each glucuronide metabolite to be analyzed.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.