810253P
Avanti
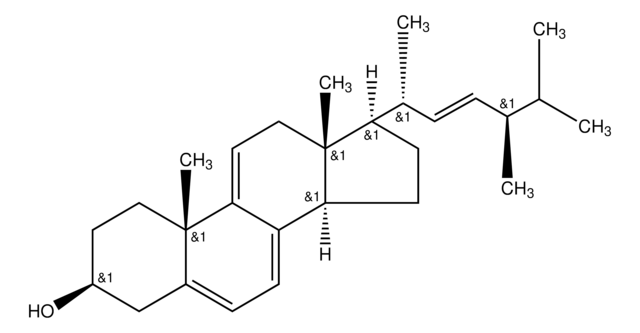
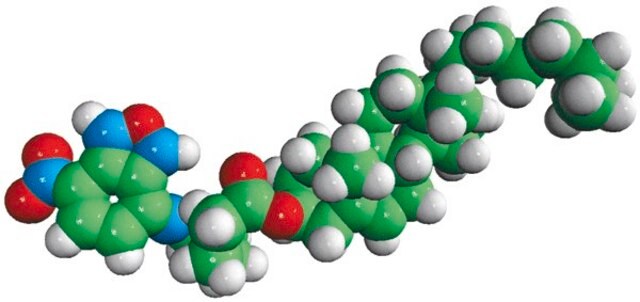
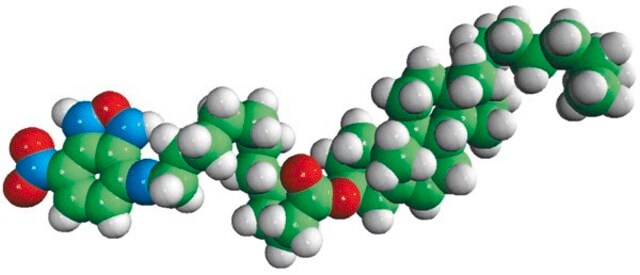
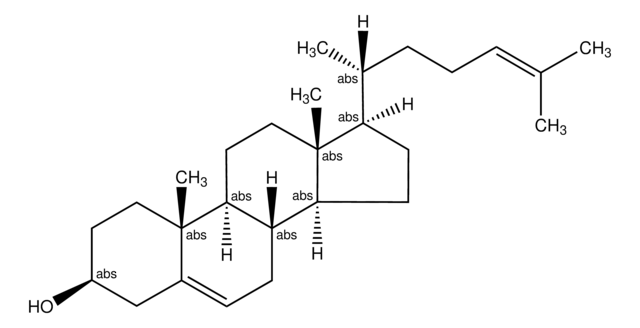
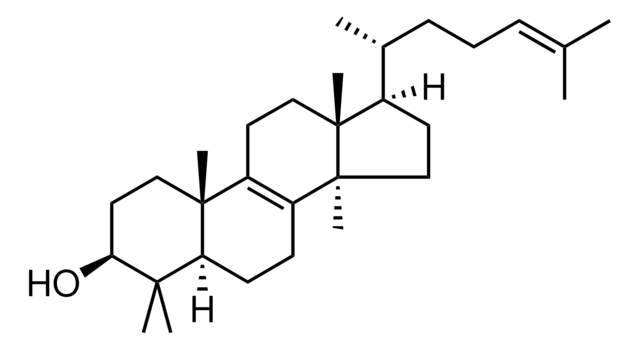
dehydroergosterol (DHE)
ergosta-5,7,9(11),22-tetraen-3β-ol, powder
Synonym(e):
DHE
About This Item
Empfohlene Produkte
Assay
>99% (TLC)
Form
powder
Verpackung
pkg of 1 × 1 mg (with stopper and crimp cap (810253P-1mg))
Hersteller/Markenname
Avanti Research™ - A Croda Brand 810253P
Versandbedingung
dry ice
Lagertemp.
−70°C
SMILES String
O[C@H]1CC[C@]2(C(=CC=C3[C@H]4[C@@]([C@H](CC4)[C@H](C)\C=C\[C@@H](C(C)C)C)(CC=C32)C)C1)C
InChI
1S/C28H42O/c1-18(2)19(3)7-8-20(4)24-11-12-25-23-10-9-21-17-22(29)13-15-27(21,5)26(23)14-16-28(24,25)6/h7-10,14,18-20,22,24-25,29H,11-13,15-17H2,1-6H3/b8-7+/t19-,20+,22-,24+,25-,27-,28+/m0/s1
InChIKey
QSVJYFLQYMVBDR-CMNOFMQQSA-N
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
Biochem./physiol. Wirkung
Verpackung
Rechtliche Hinweise
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 2
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Leider sind derzeit keine COAs für dieses Produkt online verfügbar.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.