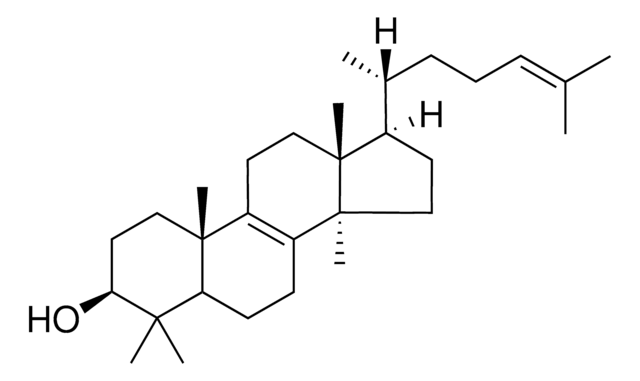
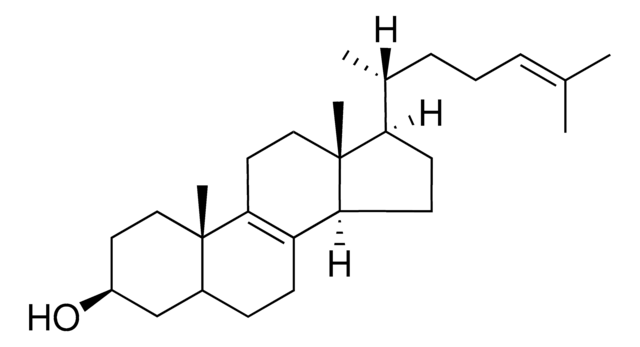
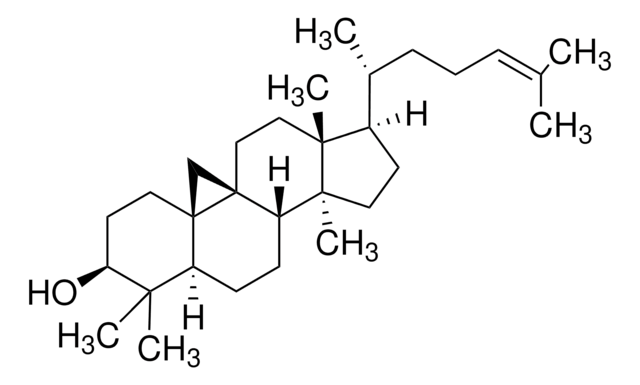
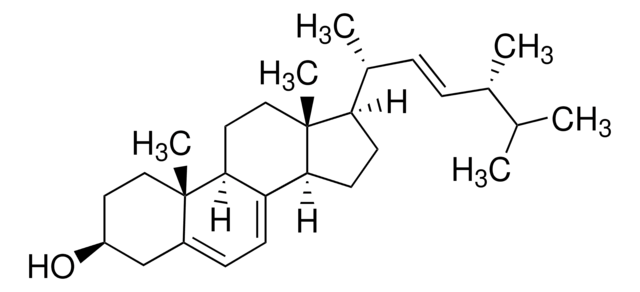
L5768
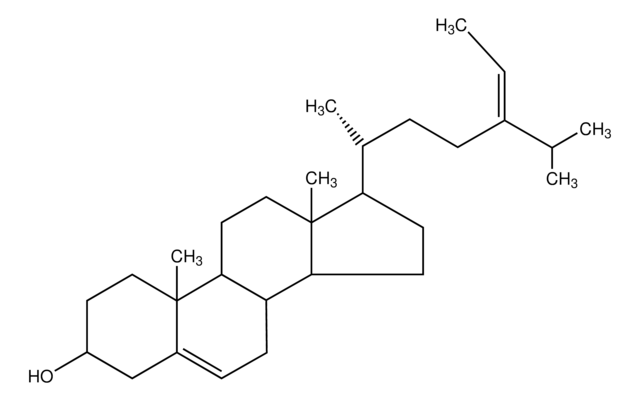
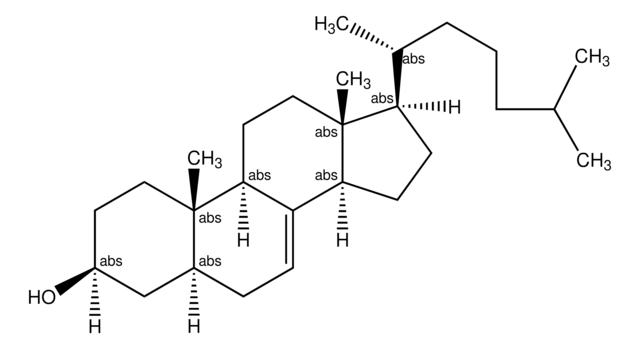
Lanosterin
≥93%, powder
Synonym(e):
3β-Hydroxy-8,24-lanostadiene, 8,24-Lanostadien-3β-ol
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥93%
Form
powder
Farbe
white to off-white
Lagertemp.
−20°C
SMILES String
[H][C@@]1(CC[C@@]2(C)C3=C(CC[C@]12C)[C@@]4(C)CC[C@H](O)C(C)(C)[C@]4([H])CC3)[C@H](C)CC\C=C(/C)C
InChI
1S/C30H50O/c1-20(2)10-9-11-21(3)22-14-18-30(8)24-12-13-25-27(4,5)26(31)16-17-28(25,6)23(24)15-19-29(22,30)7/h10,21-22,25-26,31H,9,11-19H2,1-8H3/t21-,22-,25+,26+,28-,29-,30+/m1/s1
InChIKey
CAHGCLMLTWQZNJ-BQNIITSRSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- as a standard in HPLC for the quantification in testis samples
- in S-adenosyl-L-methionine:Δ24-sterol-C-methyltransferase (SMT) assay
- to treat wild-type cells growing in rich medium to know its effects on Sre1 protein
Biochem./physiol. Wirkung
Lanosterol serves as an endogenous selective modulator of macrophage immunity.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 1
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Biosynthesis of cholesterol generally takes place in the endoplasmic reticulum of hepatic cells and begins with acetyl- CoA, which is mainly derived from an oxidation reaction in the mitochondria. Acetyl-CoA and acetoacetyl-CoA are converted to 3-hydroxy- 3-methylglutaryl-CoA (HMG-CoA) by HMG-CoA synthase.
Verwandter Inhalt
Discover Bioactive Small Molecules for Lipid Signaling Research
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.