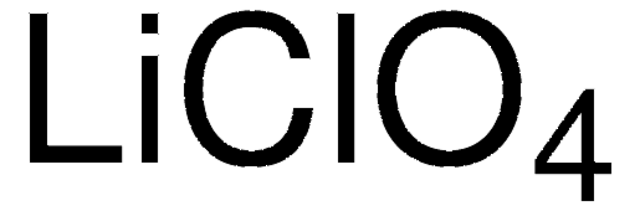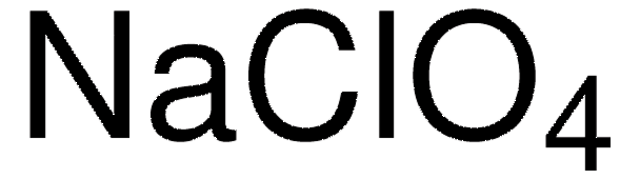931969
Lithiumperchlorat
anhydrous, ≥99.9% trace metals basis
Synonym(e):
Perchloric acid lithium salt
About This Item
Empfohlene Produkte
Qualität
anhydrous
battery grade
Qualitätsniveau
Assay
≥99.9% trace metals basis
Form
powder
Grünere Alternativprodukt-Eigenschaften
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Verunreinigungen
≤1000 ppm (trace metals analysis)
pH-Wert
6.0-7.5 (25 °C, 5%, aq.sol.)
mp (Schmelzpunkt)
236 °C (lit.)
Löslichkeit
H2O: 59.8 g/dL at 25 °C
Anionenspuren
chloride (Cl-): ≤30 ppm
sulfate (SO42-): ≤10 ppm
Kationenspuren
Fe: ≤5 ppm
heavy metals: ≤10 ppm
Anwendung(en)
battery manufacturing
Grünere Alternativprodukt-Kategorie
SMILES String
[Li+].[O-]Cl(=O)(=O)=O
InChI
1S/ClHO4.Li/c2-1(3,4)5;/h(H,2,3,4,5);/q;+1/p-1
InChIKey
MHCFAGZWMAWTNR-UHFFFAOYSA-M
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Industrially, lithium perchlorate is manufactured in several ways. Most commonly, it is prepared from sodium perchlorate through a metathesis reaction with lithium chloride or lithium carbonate. Lithium perchlorate can also be prepared by direct electrochemical oxidation of lithium chloride or by reacting lithium carbonate with perchloric acid. The hydrate can be dried either by highly controlled heating or by displacing water with volatile amines, which are removed by drying under vacuum.
Anwendung
Researchers also use lithium perchlorate as an electrolytic salt in aqueous media when testing electrocatalysts. For example, recent experiments improving the electrochemical reduction of nitrogen over TiO2 nanoparticles or gold nanoparticles use aqueous lithium perchlorate as the electrolyte.
Verpackung
500g in poly bottle
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1A - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 1
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Leider sind derzeit keine COAs für dieses Produkt online verfügbar.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.