Alle Fotos(2)
Wichtige Dokumente
792764
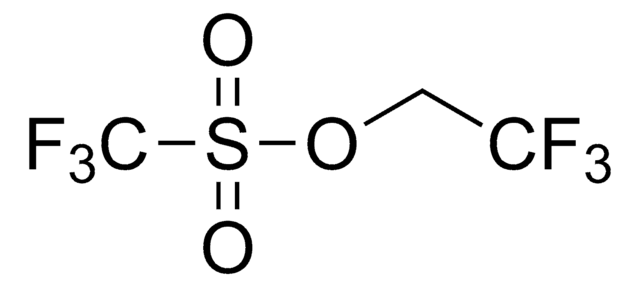
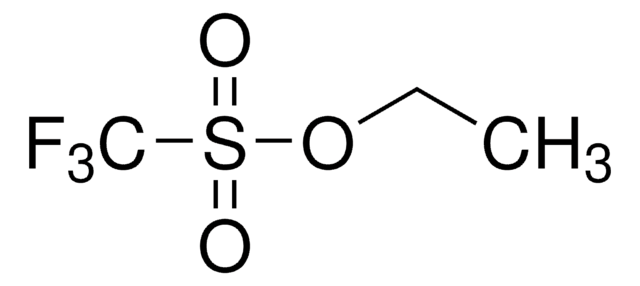
Difluoromethyl triflate
95%
Synonym(e):
Difluoromethyl trifluoromethanesulfonate, Trifluoromethanesulfonic acid difluoromethyl ester
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C2HF5O3S
CAS-Nummer:
Molekulargewicht:
200.08
MDL-Nummer:
UNSPSC-Code:
12352108
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
95%
Form
liquid
Dichte
1.584 g/mL at 25 °C
Funktionelle Gruppe
fluoro
triflate
Lagertemp.
2-8°C
SMILES String
O=S(OC(F)F)(C(F)(F)F)=O
InChI
1S/C2HF5O3S/c3-1(4)10-11(8,9)2(5,6)7/h1H
InChIKey
DAANAKGWBDWGBQ-UHFFFAOYSA-N
Allgemeine Beschreibung
Difluoromethyl triflate (HCF2OTf) is an easy to handle, air-stable and non-ozone-depleting liquid reagent for difluoromethylation. It can be prepared by reacting trifluoromethyltrimethylsilane (TMSCF3) and triflic acid in the presence of titanium tetrachloride (TiCl4).
Anwendung
Difluoromethyl triflate (TfO-CHF2) can be used as a reagent:
It allows for a simple method toward the preparation of difluoromethyl ethers and thioethers under basic conditions from alcohols and thiols. Difluoromethyl phenols can also be obtained in a single pot from boronic acids and C-H activation of arenes.
- In difluoromethylation reaction.
- To prepare difluoromethoxylated heterocycles by reacting with hydroxylated N-based heterocycles.
- To synthesize trifluoromethylated arenes by treating with diaryliodonium salts in the presence of copper and tetrabutylammonium difluorotriphenylsilicate (TBAT).
It allows for a simple method toward the preparation of difluoromethyl ethers and thioethers under basic conditions from alcohols and thiols. Difluoromethyl phenols can also be obtained in a single pot from boronic acids and C-H activation of arenes.
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 3
Flammpunkt (°F)
135.0 °F
Flammpunkt (°C)
57.22 °C
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Synthesis of difluoromethyl ethers with difluoromethyltriflate.
Patrick S Fier et al.
Angewandte Chemie (International ed. in English), 52(7), 2092-2095 (2013-01-12)
Difluoromethoxylation of N-Heteroaromatics
Snieckus V and Richardson P
Synfacts, 14(03), 0239-0239 (2018)
Difluoromethyl Triflate
Besset T
Encyclopedia of Reagents for Organic Synthesis, Second Edition, 203(03), 1-1 (2001)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.