666548
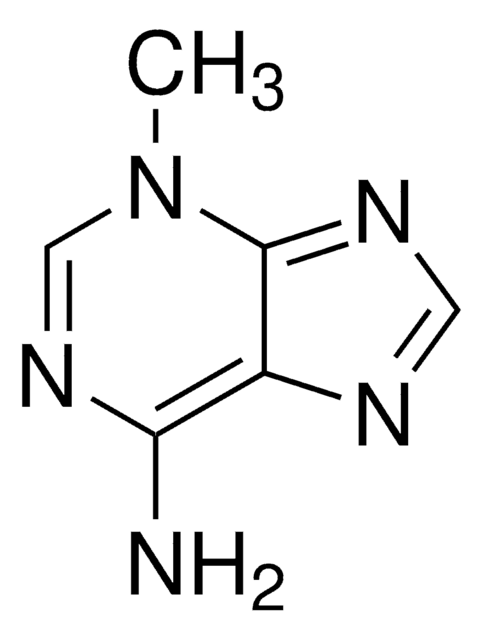
7-Methyladenin
97%
Synonym(e):
6-Amino-7-methylpurin, 7-Methyl-7H-purin-6-amin
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C6H7N5
CAS-Nummer:
Molekulargewicht:
149.15
MDL-Nummer:
UNSPSC-Code:
12352005
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
97%
Form
solid
mp (Schmelzpunkt)
346-350 °C
SMILES String
Cn1cnc2ncnc(N)c12
InChI
1S/C6H7N5/c1-11-3-10-6-4(11)5(7)8-2-9-6/h2-3H,1H3,(H2,7,8,9)
InChIKey
HCGHYQLFMPXSDU-UHFFFAOYSA-N
Anwendung
<ul>
<li><strong>Biomarkers of Cigarette Smoking and DNA Methylating Agents:</strong> Study on 7-Methyladenine highlights its role as a biomarker of DNA damage from exposure to methylating agents (Harroun et al., 2017).</li>
</ul>
<li><strong>Biomarkers of Cigarette Smoking and DNA Methylating Agents:</strong> Study on 7-Methyladenine highlights its role as a biomarker of DNA damage from exposure to methylating agents (Harroun et al., 2017).</li>
</ul>
Verpackung
Bottomless glass bottle. Contents are inside inserted fused cone.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Sens. 1
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
B Tudek et al.
Acta biochimica Polonica, 46(3), 785-799 (2000-03-04)
The most abundant lesion formed in DNA upon modification with methylating agents 7-methylguanine, under alkaline conditions is converted into 2,6-diamino-4-hydroxy-5N-methyl-formamidopyrimidine (Fapy-7MeGua). We have previously shown that treatment of dimethylsulfate methylated DNA with NaOH creates mutagenic base derivatives leading to a
H G Mandel et al.
Analytical biochemistry, 217(2), 292-297 (1994-03-01)
We have developed a procedure for isolating and quantifying 7-methyladenine from rat urine following the administration to the rat of methylating agents, such as dimethylnitrosamine. Urinary 7-methyladenine and its trideutero isomer, added as an internal standard, were precipitated with silver
Shinji Tokuda et al.
Bioscience, biotechnology, and biochemistry, 76(4), 828-830 (2012-04-10)
Adenine had a concentration-dependent relaxation action on the phenylephrine-contracted aorta ring, with an EC(50) value of 0.40±0.12 mM. This effect was also observed in the endothelium-denuded aorta. Among the adenine analogues, N-methyladenine and benzimidazole still evoked an apparent relaxation effect
H G Mandel et al.
Carcinogenesis, 15(7), 1393-1398 (1994-07-01)
Earlier studies showed that urine of rats which had been injected with the methylating agent N-[3H-methyl]-N-nitrosourea contained a previously undetected metabolic product, 7-[3H-methyl]adenine. This methylpurine, undoubtedly derived from alkylation of nucleic acids followed by depurination, was not labeled when 14C-methyl-labeled
H G Mandel et al.
Carcinogenesis, 10(4), 757-762 (1989-04-01)
Relatively simple and rapid analytical procedures involving two sequential HPLC separations were developed for the isolation of methylated purines in the urine of rats administered radiolabeled methylating carcinogens. Following a dose of [3H]N-methyl-N-nitrosourea (MNU), 7-methyl-adenine (m7Gua) was detected by chromatography
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.