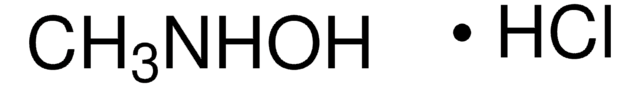
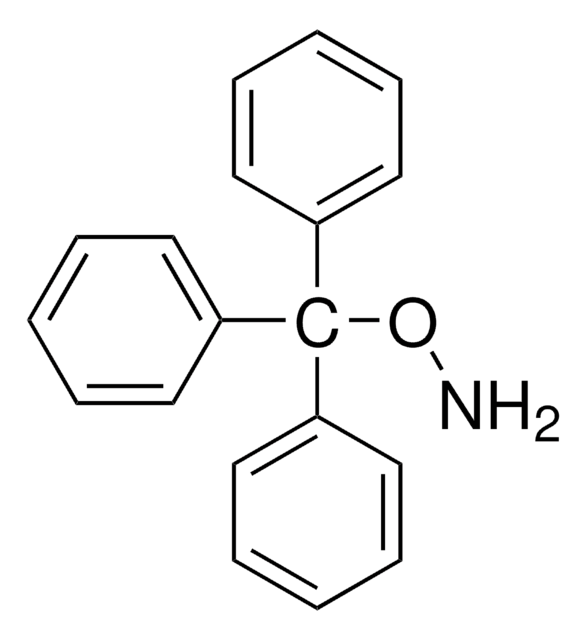
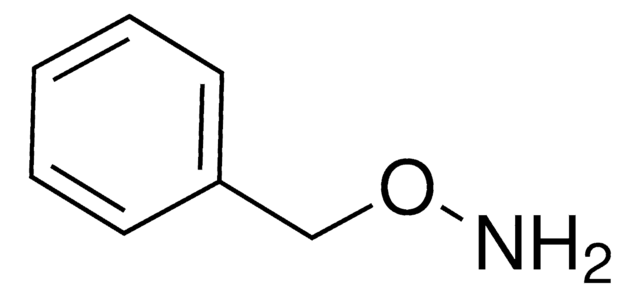
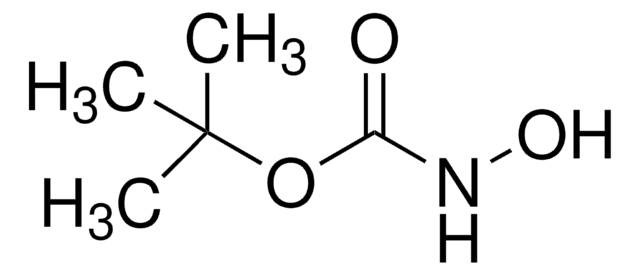
480894
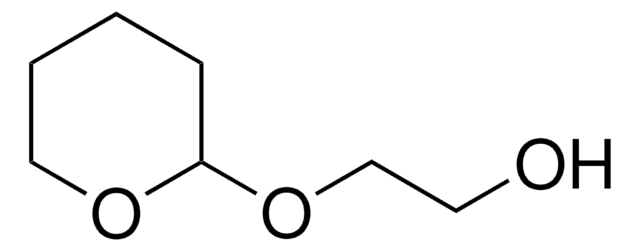
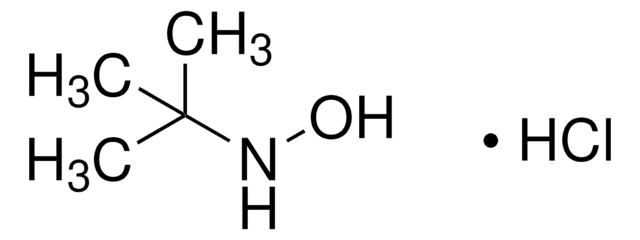
O-(Tetrahydro-2H-pyran-2-yl)hydroxylamin
96%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C5H11NO2
CAS-Nummer:
Molekulargewicht:
117.15
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
96%
bp
81 °C/20 mmHg (lit.)
mp (Schmelzpunkt)
34-37 °C (lit.)
Funktionelle Gruppe
ether
SMILES String
NOC1CCCCO1
InChI
1S/C5H11NO2/c6-8-5-3-1-2-4-7-5/h5H,1-4,6H2
InChIKey
NLXXVSKHVGDQAT-UHFFFAOYSA-N
Allgemeine Beschreibung
O-(Tetrahydro-2H-pyran-2-yl)hydroxylamine (OTX) is an O-substituted hydroxylamine. The coupling of OTX with alkaline gel electrophoresis has been reported to improve the process of detecting single strand breaks (SSBs) in DNA.
Anwendung
O-(Tetrahydro-2H-pyran-2-yl)hydroxylamine may be used in the synthesis of 2-(5-bromothiophene-2-sulfonamido)-N-(tetrahydro-2H-pyran-2-yloxy)acetamides.
It may be used in the synthesis of the following potential histone deacetylase (HDAC) inhibitors:
It may be used in the synthesis of the following potential histone deacetylase (HDAC) inhibitors:
- 2-[1-(naphthalene-2-sulfonyl)-heterocyclyl]-pyrimidine-5-carboxylic acid (tetrahydropyran-2-yloxy)-amides
- (E)-3-(2-benzyl-1-oxoisoindolin-6-yl)-N-(tetrahydro-2H-pyran-2-yloxy)acrylamide
- 3-(1-benzenesulfonyl-2,3-dihydro-1H-indol-5-yl)-N-hydroxy-acrylamide
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
179.6 °F - closed cup
Flammpunkt (°C)
82 °C - closed cup
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Chihiro Shinji et al.
Bioorganic & medicinal chemistry, 14(22), 7625-7651 (2006-08-01)
A series of hydroxamic acid derivatives bearing a cyclic amide/imide group as a linker and/or cap structure, prepared during our structural development studies based on thalidomide, showed class-selective potent histone deacetylase (HDAC)-inhibitory activity. Structure-activity relationship studies indicated that the steric
Patrick Angibaud et al.
European journal of medicinal chemistry, 40(6), 597-606 (2005-06-01)
A series of pyrimidyl-5-hydroxamic acids was prepared for evaluation as inhibitors of histone deacetylase (HDAC). Amino-2-pyrimidinyl can be used as a linker to provide HDAC inhibitors of good enzymatic potency.
Elisa Nuti et al.
European journal of medicinal chemistry, 46(7), 2617-2629 (2011-04-26)
Matrix metalloproteinases (MMPs) are important factors in gliomas since these enzymes facilitate invasion into the surrounding brain and participate in neovascularization. In particular, the gelatinases (MMP-2 and MMP-9), and more recently MMP-25, have been shown to be highly expressed in
Tetrahedron Letters, 45, 133-133 (2004)
Han-Li Huang et al.
PloS one, 7(8), e43645-e43645 (2012-08-29)
Recently, histone deacetylase (HDAC) inhibitors have emerged as a promising class of drugs for treatment of cancers, especially subcutaneous T-cell lymphoma. In this study, we demonstrated that MPT0E028, a novel N-hydroxyacrylamide-derived HDAC inhibitor, inhibited human colorectal cancer HCT116 cell growth
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.