457701
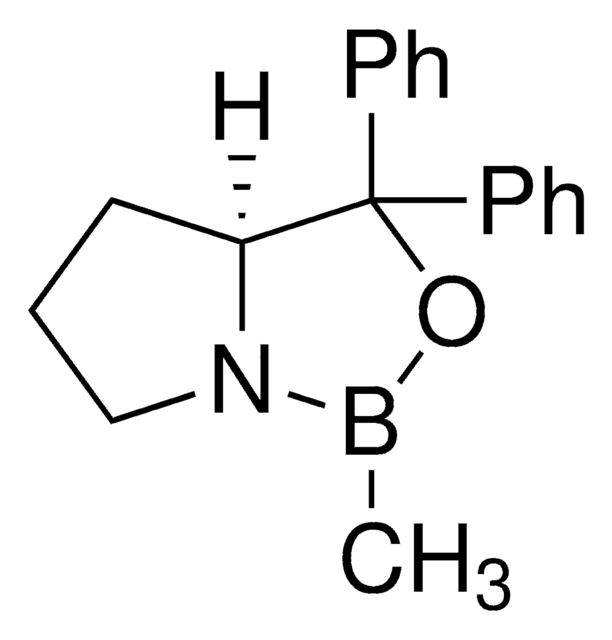
(S)-Tetrahydro-1-methyl-3,3-diphenyl-1H,3H-pyrrolo[1,2-c][1,3,2]oxazaborol -Lösung
1 M in toluene
Synonym(e):
α,α-Diphenyl-L-prolinolmethylboronsäure-cycl-amidester, (S)-(−)-2-Methyl-CBS-oxazaborolidin, (S)-1-Methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2c][1,3,2]oxazaborol, (S)-3,3-Diphenyl-1-methyltetrahydro-3H-pyrrolo[1,2-c][1,3,2]oxazaborol
About This Item
Empfohlene Produkte
Qualitätsniveau
Konzentration
1 M in toluene
bp
111 °C
Dichte
0.929 g/mL at 25 °C
Funktionelle Gruppe
phenyl
Lagertemp.
room temp
SMILES String
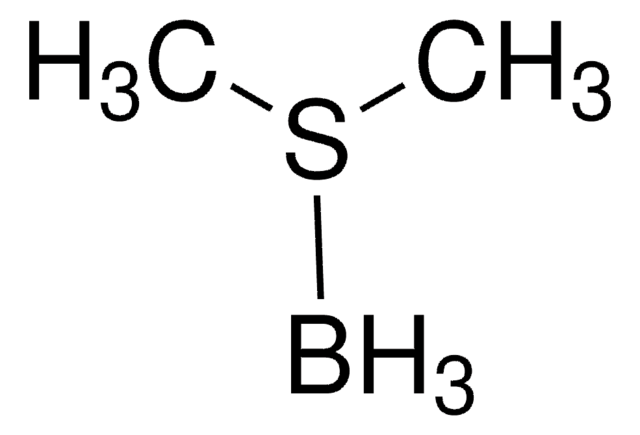
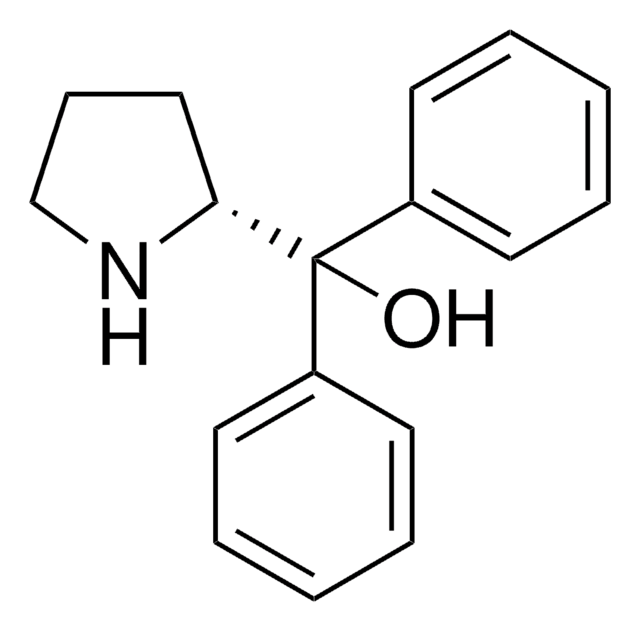
CB1OC([C@@H]2CCCN12)(c3ccccc3)c4ccccc4
InChI
1S/C18H20BNO/c1-19-20-14-8-13-17(20)18(21-19,15-9-4-2-5-10-15)16-11-6-3-7-12-16/h2-7,9-12,17H,8,13-14H2,1H3/t17-/m0/s1
InChIKey
VMKAFJQFKBASMU-KRWDZBQOSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
It may also be used in the preparation of:
- (1S)-2-azido-1-(2,2-dimethyl-4H-1,3-benzodioxin-6-yl)ethanol
- (R)-α-deuteriobenzyl alcohol
- (R)-2-(1-hydroxyethyl)benzo[b]thiophene
Sonstige Hinweise
Signalwort
Danger
Gefahreneinstufungen
Acute Tox. 4 Oral - Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 2 - STOT SE 3
Zielorgane
Central nervous system
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 3
Flammpunkt (°F)
39.2 °F - closed cup
Flammpunkt (°C)
4 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
We are pleased to offer o-tolyl-CBS-oxazaborolidine as a 0.5 M solution in toluene for your research needs. When protonated with trifluoromethanesulfonimide, these chiral oxazaborolidines generate chiral Lewis acids, which have demonstrated great utility in the enantioselective Diels–Alder reaction.
we are pleased to offer both enatiomers of 2-methyl-CBS-oxazaborolidine as a dry reagent, in addition to our current offerings as a 1M solution in toluene.
Verwandter Inhalt
Our company is pleased to offer both enantiomers of 2-methyl-CBS-oxazaborolidine as a dry reagent, in addition to our current offerings as a 1 M solution in toluene.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.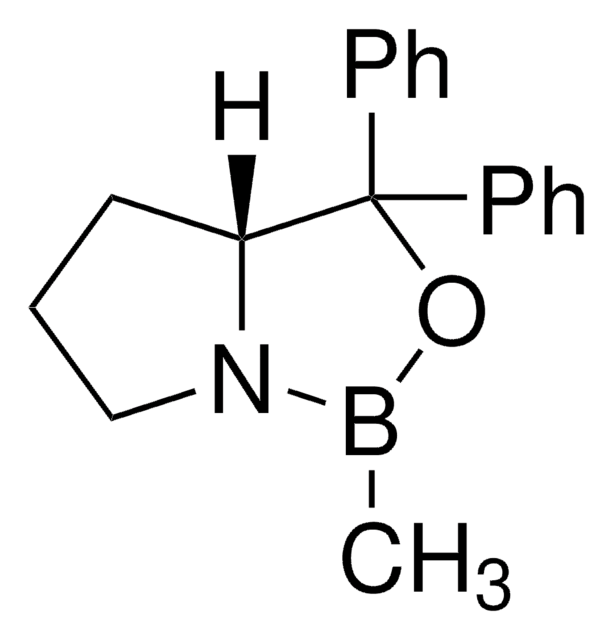
![(S,S)-(+)-2,6-Bis[2-(hydroxydiphenylmethyl)-1-pyrrolidinyl-methyl]-4-Methylphenol 95%](/deepweb/assets/sigmaaldrich/product/structures/126/939/bff1a61c-8335-434f-8da4-9b1929aef17f/640/bff1a61c-8335-434f-8da4-9b1929aef17f.png)




