Alle Fotos(1)
Wichtige Dokumente
147400
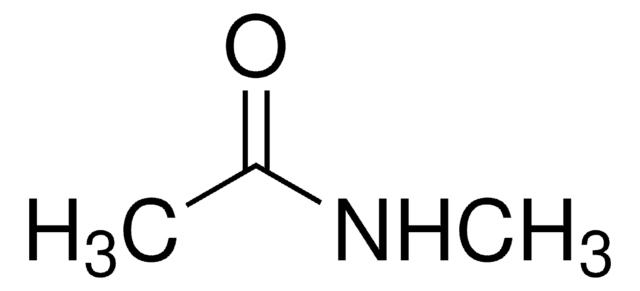
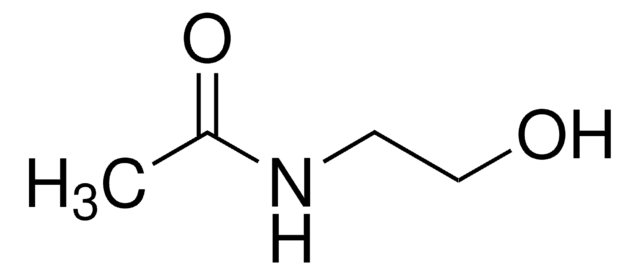
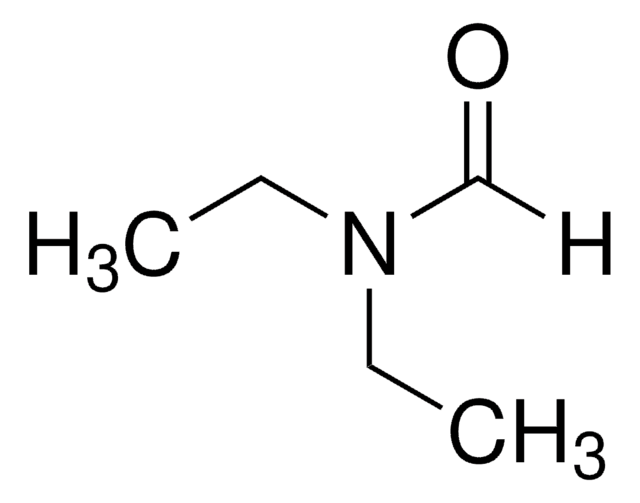
N-Ethylacetamid
99%
Synonym(e):
Acetamidoethane, N-Acetylethylamine
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
CH3CONHC2H5
CAS-Nummer:
Molekulargewicht:
87.12
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
99%
Form
liquid
Brechungsindex
n20/D 1.433 (lit.)
bp
90-92 °C/8 mmHg (lit.)
Dichte
0.924 g/mL at 25 °C (lit.)
Funktionelle Gruppe
amide
SMILES String
CCNC(C)=O
InChI
1S/C4H9NO/c1-3-5-4(2)6/h3H2,1-2H3,(H,5,6)
InChIKey
PMDCZENCAXMSOU-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
N-Ethylacetamide is a suitable nylon model for mechanistic studies of dye fading.
Anwendung
N-Ethylacetamide was used to investigate propylene carbonate and a mixture of N-methylformamide and N-ethylacetamide by broadband dielectric and mechanical shear spectroscopy. It was used in determination of reaction products of OH-oxidation of N-methylpyrrolidone under atmospheric conditions.
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Jolanta Świergiel et al.
Physical chemistry chemical physics : PCCP, 13(9), 3911-3916 (2011-01-07)
The impedance spectroscopy studies performed for two strongly hydrogen-bonded liquid amides: N-methylpropionamide (NMP, CH(3)·NH·CO·C(2)H(5)) and N-ethylacetamide (NEA, C(2)H(5)·NH·CO·CH(3)) have shown that the two centers of the peptide linkage, -NH·CO-, active in the C=OH-N hydrogen bonds formation, exhibit quite different sensibilities
Catalin Gainaru et al.
The Journal of chemical physics, 137(6), 064508-064508 (2012-08-18)
Propylene carbonate and a mixture of two secondary amides, N-methylformamide and N-ethylacetamide, are investigated by means of broadband dielectric and mechanical shear spectroscopy. The similarities between the rheological and the dielectric responses of these liquids and of the previously investigated
Tetsuya Tatsukawa et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 26(18), 4820-4825 (2006-05-05)
AMPA receptor (AMPAR) internalization provides a mechanism for long-term depression (LTD) in both hippocampal pyramidal neurons and cerebellar Purkinje cells (PCs). Cerebellar LTD at the parallel fiber (PF)-PC synapse is the underlying basis of motor learning and requires AMPAR activation
Li-Min Wang et al.
The Journal of chemical physics, 123(5), 054516-054516 (2005-08-20)
Dielectric relaxation dynamics of secondary amides is explored in their supercooled state near the glass transition temperature Tg by investigating N-ethylacetamide and its mixtures with N-methylformamide. All the samples are found to exhibit giant dielectric permittivities, reaching over 500 in
R Buchet et al.
Biophysical chemistry, 22(4), 249-254 (1985-10-01)
It is shown that a striking parallelism exists between the anesthetic potency of general halocarbon anesthetics and their influence on the hydrogen bond association constants in N-H...O=C type hydrogen bonds, important for shaping the ion channels. It is further shown
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.