Wichtige Dokumente
M26305
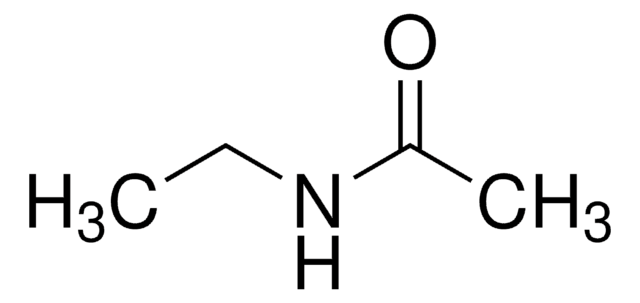
N-Methylacetamid
≥99%
Synonym(e):
Acetylmethylamin
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥99%
Brechungsindex
n20/D 1.433 (lit.)
bp
204-206 °C (lit.)
mp (Schmelzpunkt)
26-28 °C (lit.)
Dichte
0.957 g/mL at 25 °C (lit.)
SMILES String
CNC(C)=O
InChI
1S/C3H7NO/c1-3(5)4-2/h1-2H3,(H,4,5)
InChIKey
OHLUUHNLEMFGTQ-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
- To synthesize N-methyl-N-(3-thienyl)acetamide by reacting with 3-bromothiophene in the presence of CuI catalyst and N,N′-dimethylethylenediamine.(1)
- As a ligand to synthesize the zirconium(IV) complex, Zr(MeC(O)NMe)4 by reacting with tetrakis(dimethylamido)zirconium.(2)
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Repr. 1B
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flammpunkt (°F)
240.8 °F
Flammpunkt (°C)
116 °C
Persönliche Schutzausrüstung
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Zulassungslistungen
Zulassungslistungen werden hauptsächlich für chemische Produkte erstellt. Für nicht-chemische Produkte können hier nur begrenzte Angaben gemacht werden. Kein Eintrag bedeutet, dass keine der Komponenten gelistet ist. Es liegt in der Verantwortung des Benutzers, die sichere und legale Verwendung des Produkts zu gewährleisten.
EU REACH SVHC Candidate List
EU REACH Annex XVII (Restriction List)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.