135992
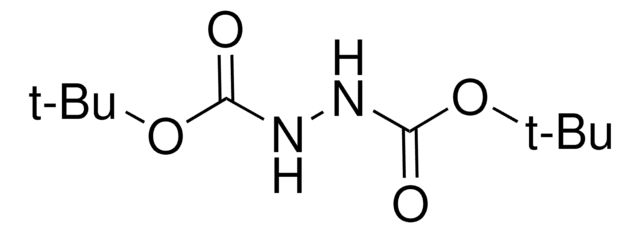
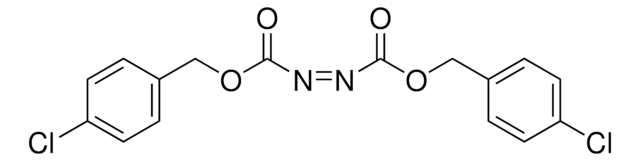
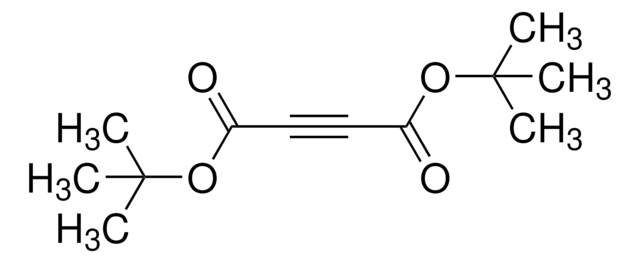
Azodicarbonsäure-di-tert.-butylester
98%
Synonym(e):
Bis(1,1-dimethylethyl)azodicarboxylate, DBAD, Di-tert-butyl azodiformate, NSC 109889
About This Item
Empfohlene Produkte
Assay
98%
Form
solid
Grünere Alternativprodukt-Eigenschaften
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp (Schmelzpunkt)
89-92 °C (lit.)
Grünere Alternativprodukt-Kategorie
Lagertemp.
2-8°C
SMILES String
CC(C)(C)OC(=O)\N=N\C(=O)OC(C)(C)C
InChI
1S/C10H18N2O4/c1-9(2,3)15-7(13)11-12-8(14)16-10(4,5)6/h1-6H3/b12-11+
InChIKey
QKSQWQOAUQFORH-VAWYXSNFSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Automate your Mitsunobu reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Anwendung
Modified Markó′s aerobic oxidation of alcohols under atmospheric pressure with air or molecular oxygen at room temperature
- Preparation of hexapeptide key fragments via stereoselective selenocyclization/oxidative deselenylation or hydrazination/cyclization reactions
- Asymmetric Michael addition reactions
- Preparation of dipeptidyl peptidase IV dependent water-soluble prodrugs via Mitsunobu reaction
- Synthesis of pyrroloisoquinoline template via stereoselective N-acyliminium-mediated cyclization and enolate amination for synthesis of peptidomimetic compounds
- Barbier-type propargylation reactions
- Synthesis of bacterial peptide deformylase (PDF) inhibitor fumimycin
- Asymmetric amination of glycine Schiff bases
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
One of the most powerful and widely used carboncarbon bond forming reactions in organic synthesis is the Mitsunobu reaction.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.