PHL80431
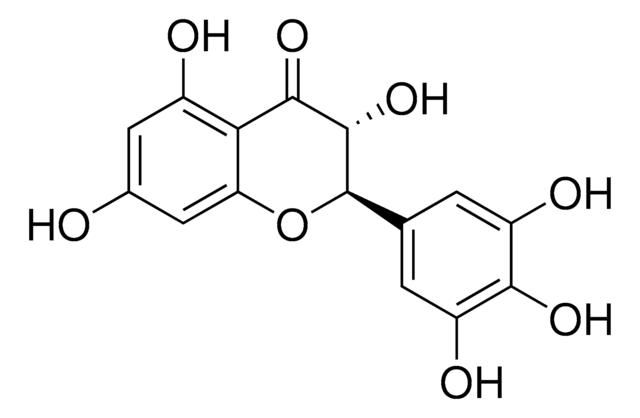
Dihydromyricetin
phyproof® Reference Substance
Sinonimo/i:
(2R,3R)-3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl)-2,3-dihydrochromen-4-one, 3,3′,4′,5,5′,7-Hexahydroxyflavanone, Ampelopsin, Ampeloptin, DHM
About This Item
Prodotti consigliati
Grado
primary reference standard
Nome Commerciale
phyproof® Reference Substance
Saggio
≥95.0% (HPLC)
Stato
solid
Produttore/marchio commerciale
PhytoLab
Stringa SMILE
O=C1C2=C(O)C=C(O)C=C2O[C@H](C3=CC(O)=C(C(O)=C3)O)[C@H]1O
InChI
1S/C15H12O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,14-20,22H/t14-,15+/m0/s1
KJXSIXMJHKAJOD-LSDHHAIUSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- Hovenia dulcis: a Chinese medicine that plays an essential role in alcohol-associated liver disease. This review discusses the role of Hovenia dulcis, from which dihydromyricetin is derived, in treating alcohol-associated liver conditions, highlighting its mechanisms and therapeutic potentials (He YX, Liu MN, Wang YY, et al. 2024).
- Dihydromyricetin ameliorates hepatic steatosis and insulin resistance via AMPK/PGC-1α and PPARα-mediated autophagy pathway. This study explores how dihydromyricetin influences liver health, particularly in hepatic steatosis and insulin resistance, offering insights into its mechanisms through autophagy pathways (Yang Y, Qiu W, Xiao J, et al. 2024).
- Identification of dihydromyricetin as a natural DNA methylation inhibitor with rejuvenating activity in human skin. Research identifies dihydromyricetin′s potential anti-aging effects on human skin by modulating DNA methylation, which could contribute to its broader use in dermatological products (Falckenhayn C, Bienkowska A, Söhle J, et al. 2023).
- Dihydromyricetin reverses capecitabine-induced peripheral myelin dysfunction through modulation of oxidative stress. This article provides evidence of dihydromyricetin′s protective effects against peripheral myelin damage due to oxidative stress, relevant in the treatment of certain neuropathies (Fang J, Lou S, Zhou X, et al. 2024).
- The Molecular Mechanism Underlying the Therapeutic Effect of Dihydromyricetin on Type 2 Diabetes Mellitus Based on Network Pharmacology, Molecular Docking, and Transcriptomics. This comprehensive study details the molecular interactions and pathways through which dihydromyricetin could affect type 2 diabetes, providing a foundation for its application in metabolic disorder treatments (Wen X, Lv C, Zhou R, et al. 2024).
Azioni biochim/fisiol
Note legali
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.